Contact
Prof. Dr. Karl-Wilhelm Koch
Tel.: +49-(0)441-798-3640
Office: W4 1-137
E-mail: karl.w.koch@uol.de
Biosensory systems
Biosensory systems

Surface plasmon resonance (SPR) spectroscopy is a biosensor technology, that uses the detection of changes in mass in an evanescent wave field (Figure 4). Biomolecules like proteins, peptides and nucleic acids are immobilized on the surface of a sensor chip and possible binding partners are flushed over the surface. Interactions with the immobilized binding partner are detected by a change in refractive index close to the chip surface. Time-courses of these changes are monitored and displayed as sensorgrams and allow the determination of kinetic parameters. Current projects are:
- Molecular mechanisms in myristoyl-switch sensor proteins
- Immobilization of phospholipid layers on sensor chips to study membrane proteins
- Investigation of G-protein coupled receptors and protein complexes on surface-modified sensorchips
Principle of surface plasmon resonance
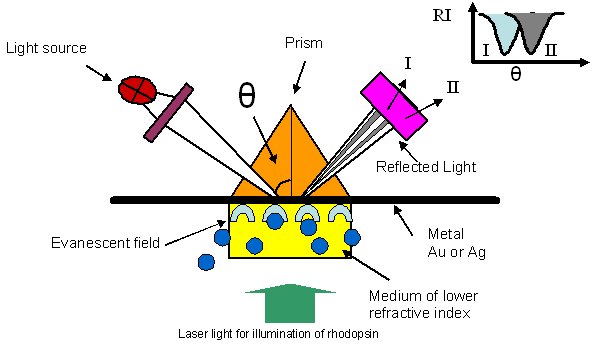
Figure 4: Principle of surface plasmon resonance. A light source emits monochromatic light that traverses through a prism at a certain angle θ and that is totally internally reflected at an interface of two media. A thin metal layer is at the boundary between the prism and a medium of lower refractive index (e.g. aqueous buffer). Interaction of the evanescent electric field and the electron constellations of the metal causes a reduction in the reflected light intensity (RI). This is illustrated as a gray shadow in the reflected light beam and as a decrease of RI in the graph RI versus θ . Changes of the dielectric properties in the vicinity of the metal surface changes the resonance conditions and leads to a shift of θ (I --> II).
Selected publications
Koch, K.-W. (2000) Identification and Characterization of Calmodulin Binding Sites in the cGMP-Gated Channel Using Surface Plasmon Resonance Spectroscopy. Meth. Enzymol. 315, 785-797
Komolov, K. E. and Koch, K.-W. (2010) Application of surface plasmon resonance spectroscopy to study G-protein coupled receptor signalling. Methods Mol. Biol. 627:249-60
Dell'Orco, D., Müller, M. and Koch, K.-W. (2010) Quantitative detection of conformational transitions in a calcium sensor protein by surface plasmon resonance. ChemCommun. 46, 7316-7318
Dell'Orco, D., Sulmann, S., Linse, S. and Koch, K.-W. (2012) Dynamics of conformational Ca2+-switches in signaling networks detected by a planar plasmonic device. Anal. Chem. 84, 2982-2989.
Sulmann, S., Dell´Orco, D., Marino, V., Behnen, P. and Koch, K.-W. (2014) Conformational changes in calcium-sensor proteins under molecular crowding conditions. Chemistry Eur. J. 20, 6756-6762.
Dell'Orco, D. and Koch, K.-W. (2016) Fingerprints of Calcium-Binding Protein Conformational Dynamics Monitored by Surface Plasmon Resonance. ACS Chem. Biol. 11, 2390-2397.
Sulmann, S., Kussrow, A., Bornhop, D.J. and Koch, K.-W. (2017) Label-free quantification of calcium-sensor targeting to photoreceptor guanylate cyclase and rhodopsin kinase by backscattering interferometry. Sci. Rep. 7:45515.

