Contact
Lab Head
Postal address
Projects
Main questions of our research:
Ultrafast temporal processing: How does the inner ear achieve this?
Hearing is the fastest of all senses. Nerve fibres carrying auditory information from the sensory hair cells of the inner ear to the brain, convey auditory signals with unparalleled temporal precision. This is true for both animals and humans. The most extreme performance is found in the barn owl, a nocturnal bird of prey. Via neural phase locking, auditory nerve fibres in the owl encode the timing of a stimulus with a precision of about 30 microseconds.
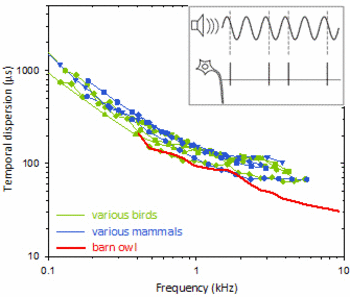
Temporal dispersion (or jitter) of phase coding in the auditory nerve as a function of stimulus frequency. Inset shows schematically how spike timing is measured. The coloured curves show median values for large samples of single-unit data in different species of birds and mammals. Note the scaling in microseconds - this degree of temporal precision is an amazing feat of the auditory system! The barn owl (red curve) holds the record, with temporal dispersions below 30µs.
Got curious? Listen to a 15-minute talk by Christine Köppl about „The amazing barn owl cochlea”. This talk was part of a symposium on Feb 17th 2021, honouring the life and work of Prof. Masakazu (Mark) Konishi, .
How are ribbon synapses involved in that?
Although well documented, it is still unknown how that kind of temporal precision is achieved. The chemical synapses between a hair cell and its afferent fibres - called ribbon synapses – are believed to play a crucial role but their exact contribution remains unclear.
We take a comparative approach and currently look at details of the ribbon synapses in the barn owl, the animal most specialised for ultrafast temporal processing, and in the chicken, a close relative with average capabilities. In addition, we are developing an electrophysiological quick test for evaluating temporal coding in birds, to be able to compare different species or animals whose inner ears have been manipulated experimentally.
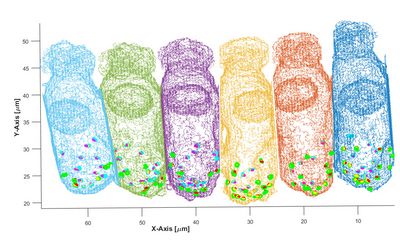
Cross-section of a few sensory hair cells from the barn owl, at very high magnification under the light microscope, focussing through successive layers in depth. Several hair cells are visible in grey. their cell nuclei in a pale red-green shade. Ribbon synapses at the bases of the hair cells are labelled by an antibody against a presynaptic protein and appear as little, red dots. The light blue structures are nerve fibres running up to the hair cells and contacting them via enlarged terminals.
What goes wrong with our hearing with advancing age?
[Supported by the Cluster of Excellence „Hearing4all”]
Humans and other mammals typically suffer from hearing problems with advancing age. This happens even without any additional damaging noise exposure, simply through ageing processes. Noise exposure, such as excessively loud music or occupational noise, makes things worse.
We focus on what exactly deteriorates with ageing in the inner ear (cochlea). Experiments on ageing gerbils aim to quantify dwindling numbers of synapses and nerve fibres providing auditory input to the brain. Electrophysiological recordings from the remaining nerve fibres look at whether their coding of the details of auditory stimuli changes.
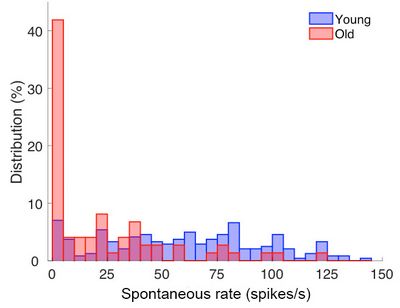
Histogram of the distribution of spontaneous discharge rates among single auditory-nerve fibres from young and old gerbils. Note that in old animals (shown in red), the discharge rates are significantly lower. In other words, the nerve fibres do not produce as many action potentials those of young animals.
Birds do not suffer age-related hearing loss
It is well known that birds are able to regenerate new hair cells after damage to their inner ears – something that mammals (and thus humans) cannot do. It is thought that these repair mechanisms also protect birds from age-related cochlear degeneration because they do not suffer hearing loss with advancing age. We have begun to investigate the inner ears of older birds (chickens and barn owls) to learn whether not just hair cells, but also the afferent and efferent nerve cells that contact them are preserved into old age.
Evolution of sound localization: How do birds localize sound?
Animals and humans use minute differences in the timing and intensity of what is heard in both ears to localise a sound in the environment: When sound comes from one side, it reaches one ear before the other and it is louder in that ear. These so-called interaural differences are measured by the brain, by comparing the inputs from both ears, and are used to synthesise a neural map of auditory space.
It has recently become clear that not all animals do these brain computations in the same way. We are interested in how and why different sound localization mechanisms evolved.
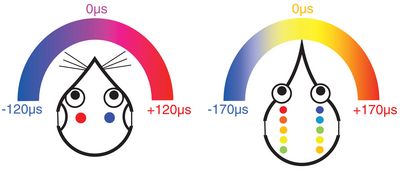
Cartoon summary of how mammals (left) and birds (right) represent interaural time differences (ITD) in their brainstem. Both examples assume typical animals with heads much smaller than a human (such as gerbil and chicken), generating a small range of ITDs, here maximally 120 and 170 microseconds. In mammals, neurone populations on each side of the brainstem are excited most when the auditory stimulus is on the contralateral side in the animal’s environment. The relative excitation of neurones on the left and right side then signals the exact location. In birds, the equivalent neurones have smaller receptive fields and the most active neurones signal the stimulus location directly.
Using the barn owl’s ITD computation circuit to learn about the sources of field potentials
Research on barn owls has a long tradition in sound localization and has contributed in particular towards understanding how the brain measures interaural time differences (ITD). The relevant brainstem neurones, their detailed morphology and connections, as well as their physiology are known in unprecedented detail in the barn owl. Together with colleagues in Berlin and Maryland (USA), we are now building on this knowledge to work out how different neuronal sources contribute to field potentials. Field potentials, such as ABR (auditory brainstem response) or EEG can also be obtained non-invasively in humans and are important for diagnosing brain problems. The better we understand what exactly generates field potentials, the better such diagnoses can be.
Molecular control of development - How does the complex inner ear differentiate?
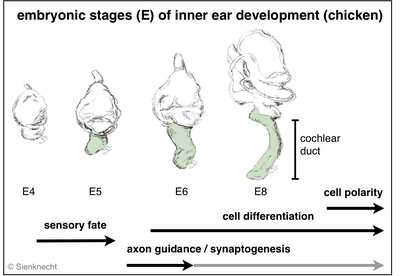
Vertebrate inner ears consist of several sensory organs (e.g. 5-6 vestibular organs in mammals and 7 in birds, respectively, in addition to the auditory organ). All develop from specified regions of the common embryonic otocyst (see diagram). The dorsal ear comprises the vestibular apparatus. Ventrally, the cochlear duct of birds and mammals houses the auditory organ and the vestibular lagena macula (although most mammals lack the latter).
Embryonic development leads to the formation of the sensory system consisting of the middle ear, the inner ear with its functional differentiations, and the neuronal projections of the individual inner-ear sensory organs to the corresponding brain stem nuclei (Fig. histological section). Multiple developmental processes are involved in this development of the inner-ear sensory system.
Such essential processes are, for instance: sensory fate specification, cell differentiation, axon guidance, and synaptogenesis. Specializations of mechanosensory cells with individual physiological and morphological properties are essential in the auditory epithelium in particular. In addition, the phenomenon of planar cell polarity (PCP) is the result of a developmental process.
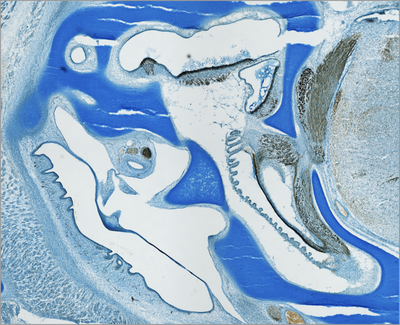
Histological section through the inner- and middle-ear region of a chicken embryo (embryonic day 11 of 21,). The inner ear develops surrounded by cartilage, the so-called otic capsule (Alcian blue staining). Immunhistochemically labeled sensory cells and neurofilaments allow to identify the different sensory organs of the inner ear and their connection to the hindbrain (brown).
How are different signaling molecules involved?
Although information has recently accumulated rapidly, our picture of the molecular network controlling these developmental processes is broadly incomplete and in many cases we don't know the role of interacting signaling pathways or genes. Thus, investigation of gene function is at the core of our interest. We perform spatio-temporal mappings of gene expression patterns (Fig. Mapping of gene expression). Moreover, we explore their role in inner ear development by gene expression gain-of-function, and loss-of-function approaches.
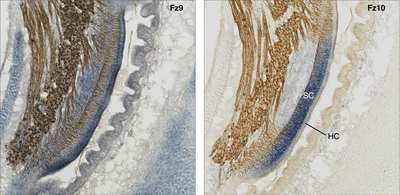
Mapping of gene expression:
Wnt signaling-related gene expression in the cochlear duct (compared are two adjacent histological sections of the same specimen). Co-labeling of the gene transcript (mRNA, blue) by in-situ hybridization (probed for two different Frizzled receptors, Fz) and neurofilament-associated antigen (brown); chicken, embryonic day 10. Note the overlapping but also partly exclusive expression pattern of the two Wnt-receptors. For instance, Fz10 is expressed in the supporting cell domain (SC) but not in the sensory cells (hair cells, Hz), whereas hair cells do express Fz9.