Group Leader
Prof. Dr. Gerhard Hilt
Secretary
Gisela Kaulfuß
Department of Chemistry (» Postal address)
Research
Research in the Hilt Group
Organic Electrochemistry
These days, the main focus of our research is directed towards the applications of “unconventional” electrochemical methods in organic electro-synthesis.
- Paired electrolysis
- Cation-pool electrolysis
- Quasi-divided cell electrolysis
- 200%-Cell electrolysis
- Alternating current electrolysis
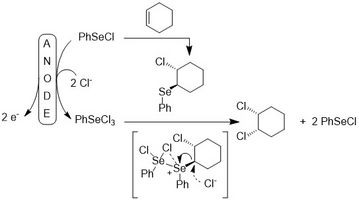
A recent example from our group is the electrochemical version of the cis-chlorination of alkenes mediated by selenium compounds.
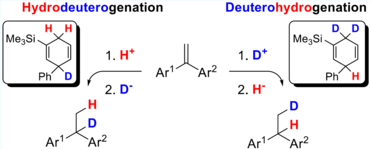
Boron- and Indium-Lewis Acid-Catalysed Transfer-Hydrogenations
The cobalt-catalysed Diels-Alder reaction allowed us to generate site-specific deuterated dihydroaromatic compounds. The transfer-hydrogenation initiated by Lewis acids, such as BF3 or InBr3 led to the regiospecific HD or DH transfer to alkenes.
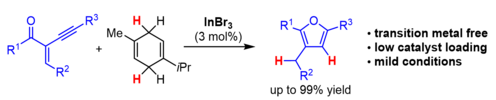
Also, an InBr3-catalysed transfer-hydrogenation initiating a cyclisation towards furan derivatives could be realised.
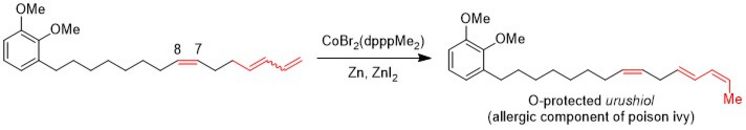
Cobalt- and Nickel-catalysed Double Bond Isomerisation
Some time ago, we identified a cobalt catalyst for the isomerisation of terminal carbon-carbon double bonds of a 1,3-diene to yield 2Z,4E-configured dienes selectively. This reaction was applied for the synthesis of the (O-protected) allergic component of poison ivy.
Thereafter, a nickel-catalyst for the selective isomerisation of terminal alkenes to the corresponding Z-2-alkenes was identified. Also, this transformation was utilised in a natural product synthesis.
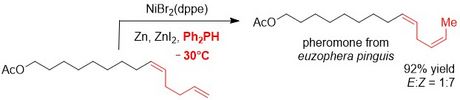
Further applications for these double bond isomerisation reactions are underway.
Cobalt-Catalysed Reactions
After two decades of research in the area of cobalt-catalysed transformations, we have left the field.
A Twenty-Year Survey in Low-Valent Cobalt-Catalyzed Transformations Comes to an End - A Farewell
Quantification of Lewis Acids and Relation to Reactivities in Organic Transformations
The quantification of Lewis acidities was an interesting side field that we have left as well.

