Group Leader
Prof. Dr. Gerhard Hilt
Secretary
Gisela Kaulfuß
Department of Chemistry (» Postal address)
Publications
2025
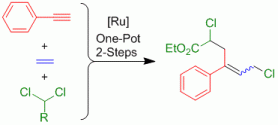
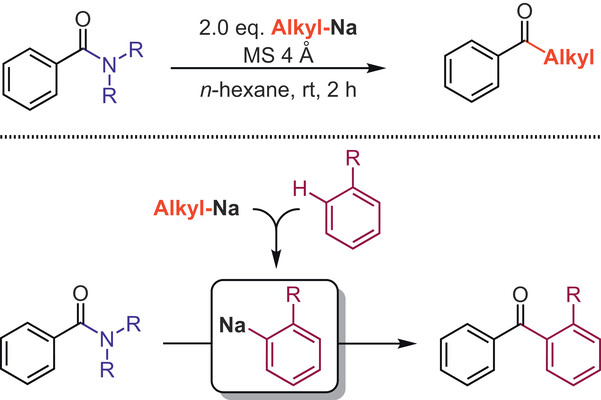
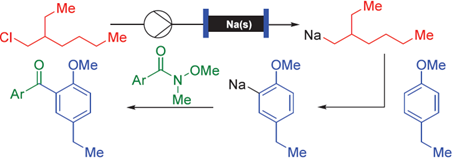
200. P. Knupe-Wolfgang, G. Hilt, Synthesis 2025; 57(21): 3263-3272, DOI: 10.1055/a-2644-2500. The Transformation of Alkyl- and Aryl-Sodium Reagents with Simple Amides for the Direct Synthesis of Ketones.
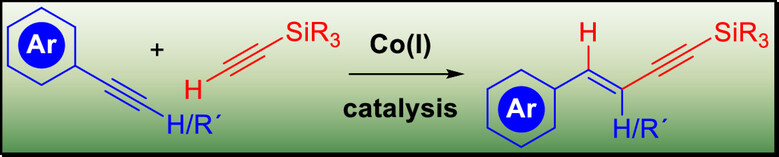
199. S. M. Weber, G. Hilt, Eur. J. Org. Chem. 2025, 28, e202500682, DOI: 10.1002/ejoc.202500682. Cobalt(I)-Catalyzed E-Selective Hydroalkynylation of Terminal Alkynes or Internal Alkynes with Silyl-Substituted Terminal Alkynes.
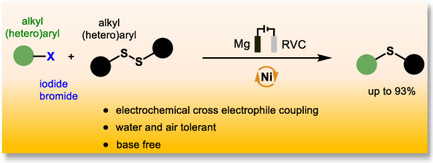
198. J. Queder, G. Hilt, Adv. Synth. Catal. 2025, 367, e70116, DOI: 10.1002/adsc.70116. Thioether Synthesis via the Nickel-Catalyzed Electrochemical Cross Electrophile Coupling of Aryl/Alkyl Disulfides and Aryl/Alkyl Halides.
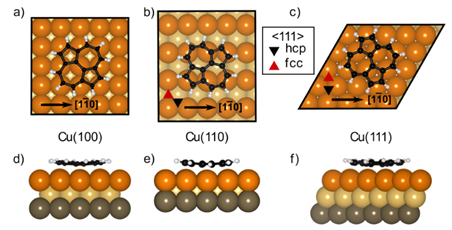
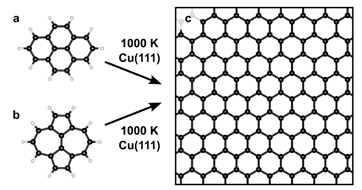
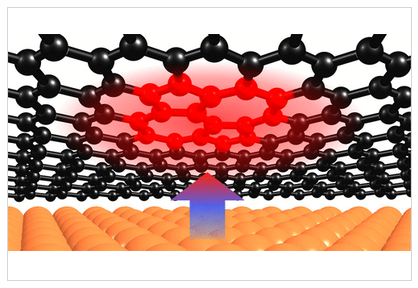
197. B. P. Klein, M. A. Stoodley, J. Deyerling, L. A. Rochford, D. B. Morgan, D. G. Hopkinson, S. Sullivan-Allsop, H. Thake, F. Eratam, L. Sattler, S. M. Weber, G. Hilt, A. Generalov, A. Preobrajenski, T. Liddy, L. B. Williams, M. Buchan, G. A. Rance, T. Lee, A. Saywell, R. Gorbachev, S. J. Haigh, C. Allen, W. Auwärter, R. Maurer and D. A. Duncan, Chem. Sci. 2025, 16, 19403-19413, DOI: 10.1039/D5SC03699B. One-step synthesis of graphene containing topological defects.
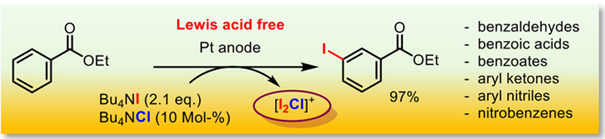
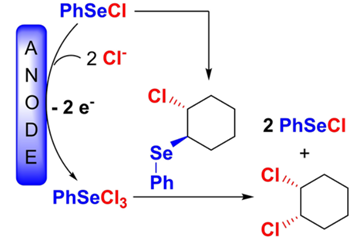
196. S. Torabi, M. Jamshidi, G. Hilt, Angew. Chem. Int. Ed. 2025, 64, e202422442; DOI: 10.1002/anie.202422442. The Electrochemical Iodination of Electron-Deficient Arenes.
2024
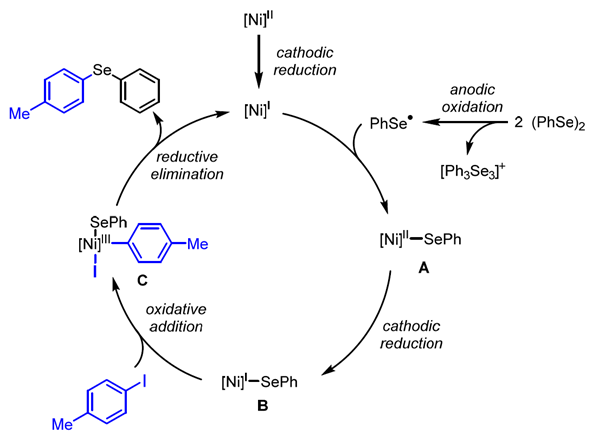
195. J. Queder, G. Hilt, Molecules 2024, 29, 4669; DOI: 10.3390/molecules29194669. Electrochemical Nickel-Catalyzed Synthesis of Unsymmetrical Diorganyl Selanes from Diaryl Diselanes and Aryl and Alkyl Iodides.
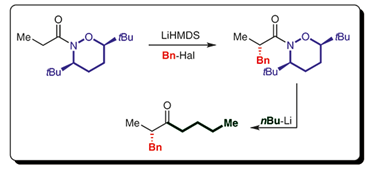
194. P. Knupe-Wolfgang, B. Mahn, G. Hilt, Org. Lett. 2024, 26, 6972-6976; DOI: 10.1021/acs.orglett.4c02314. The Application of Flow Chemistry for the Synthesis of Alkyl Sodium
Compounds and Their Transformations with Weinreb Amides and Carboxylic Acids.
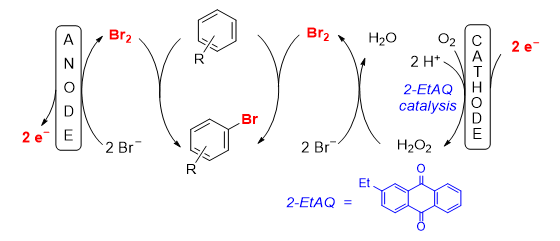
193. S. Torabi, M. Jamshidi, G. Hilt, J. Org. Chem. 2024, 89, 13953-13958; DOI: 10.1021/acs.joc.4c01086. Electrochemical Bromination of Arenes in a 200% Cell.
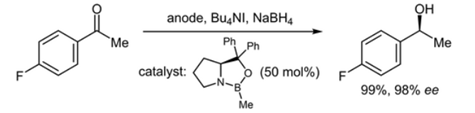
192. M. L. Abraham, G. Hilt, ChemElectroChem 2024, 11, e202400319; DOI: 10.1002/celc.202400319. Investigation Towards the Asymmetric CBS-Catalysed Reduction of Aryl Methyl Ketones with Electrochemically in Situ Generated BH3.
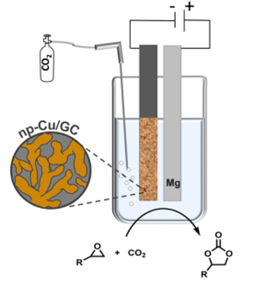
191. S. Ibrahim, D. Crespo, S. Blaseio, A. Hockmann, G. Hilt, M. Oezaslan, ChemElectroChem 2024, 11, e202400098; DOI: 10.1002/celc.202400098. Nanoporous Copper for the Electrosynthesis of Cyclic Carbonates from CO2 and Epoxides.
190. J. Queder, G. Hilt, Synlett 2024, 35, 1906-1908; DOI: 10.1055/s-0043-1763695
The Electrochemical trans-Chloroformyloxylation of Alkenes.
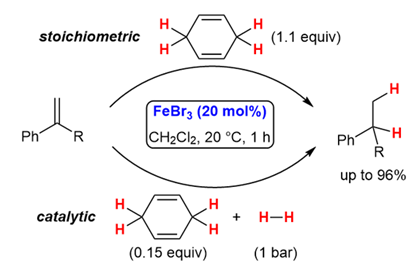
189. S. Kail. G. Hilt Synlett 2024, 35, 1011-1014; DOI: 10.1055/s-0042-1751572
The FeBr3-Catalysed Transfer Hydrogenation of Alkenes under Mild Reaction Conditions.
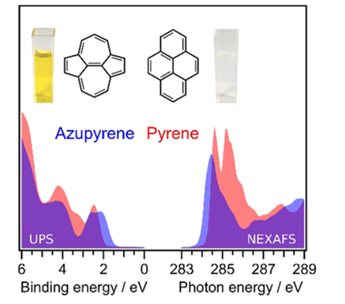
188. B. P. Klein, M. A. Stoodley, D. B. Morgan, L. A. Rochford, L. B. S. Williams, P. T. P. Ryan, L. Sattler, S. M. Weber, G. Hilt, T. J. Liddy, T.-L. Lee, R. J. Maurer, D. A. Duncan, Nanoscale 2024, 16, 5802-5812. Probing the role of surface termination in the adsorption of azupyrene on copper.
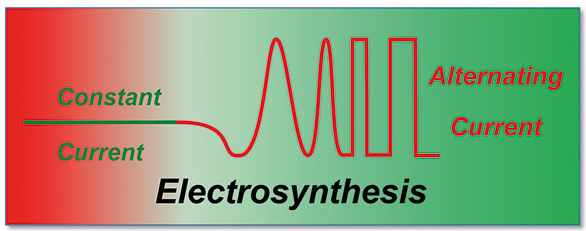
187. G. Hilt, Curr. Opin. Electrochem. 2024, 43, 101425. Recent advances in paired electrolysis and their application in organic electrosynthesis.
2023
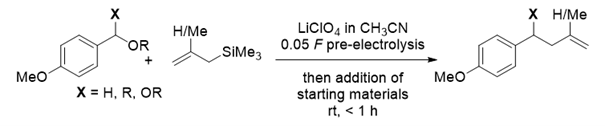
186. C. Fastie, G. Hilt, J. Org. Chem. 2023, 88, 12526-12530; DOI: 10.1021/acs.joc.3c01256
Pre-electrolysis of LiClO4 in Acetonitrile: Electrochemically Induced Protolytic Carbon–Carbon Bond Formation of Benzylic Ethers and Acetals with Allyl Trimethylsilane and Other Carbon Nucleophiles
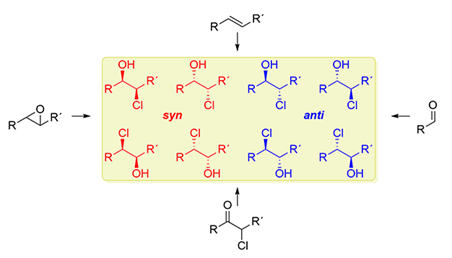
185. G. Hilt, Synthesis 2023, 54, 565-579; DOI: 10.1055/s-0042-1751379
The Synthetic Approaches to 1,2-Chlorohydrins.
184. G. Hilt, Synlett 2023, 34, 23-28; DOI: 10.1055/s-0042-1753142
A Twenty-Year Survey in Low-Valent Cobalt-Catalyzed Transformations Comes to an End - A Farewell
2022
183. B. P. Klein, M. A. Stoodley, M. Edmondson, L. A. Rochford, M. Walker, L. Sattler, S. M. Weber, G. Hilt, L. B. S. Williams, T.-L. Lee, A. Saywell, R. J. Maurer, D. A. Duncan, Appl. Phys. Lett 2022, 121, 191603; DOI: org/10.1063/5.0122914
Using polycyclic aromatic hydrocarbons for graphene growth on Cu(111) under ultra-high vaccum.
182. M. Jamshidi, C. Fastie, G. Hilt, Synthesis 2022, 54, 4661-4672; DOI: 10.1055/s-0042-1751367
Applications of Alternating Current/Alternating Potential Electrolysis in Organic Synthesis.
181. B. P. Klein, A. Ihle, S. R. Kachel, L. Ruppenthal, S. J. Hall, L. Sattler, S. M. Weber, J. Herritsch, A. Jaegermann, D. Ebeling, R. J. Maurer, G. Hilt, R. Tonner-Zech, A. Schirmeisen, J. M. Gottfried, ACS Nano 2022, 16, 11979-11987; DOI: 10.1021/acsnano.2c01952
Topological Stone-Wales Defects Enhance Bonding and Electronic Coupling at the Graphene/Metal Interface.
2021
180. J. Fährmann, L. Hermann, G. Hilt, Synthesis 2021, 54, 2005-2018; DOI: 10.1055/a-1683-0484
The Application of 1,2-Oxazines as Chiral Cyclic Weinreb-Amide Type Auxiliaries Leading to a Three-Component, One-Pot Reaction.
179. J. Strehl, C. Fastie, G. Hilt, Chem. Eur. J. 2021, 27, 17341-17345; DOI: 10.1002/chem.202103316
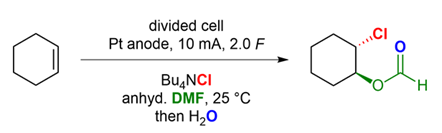
The Electrochemical cis-Chlorination of Alkenes.
178. L. Li, S. Kail, S. M. Weber, G. Hilt, Angew. Chem. 2021, 133, 23853-23858; DOI: 10.1002/ange.202109266, Angew. Chem. Int. Ed. 2021, 60, 23661-23666; DOI: 10.1002/anie.202109266
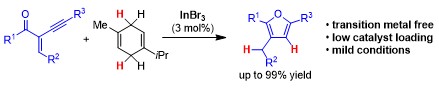
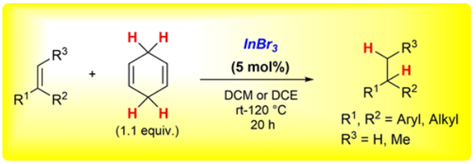
Indium-Catalysed Transfer-Hydrogenation for the Reductive Cyclisation of 2-Alkynyl Enones towards Trisubstituted Furans.
177. J. Strehl, G. Hilt, Eur. J. Org. Chem. 2022, 35-39; DOI: 10.1002/ejoc.202101007
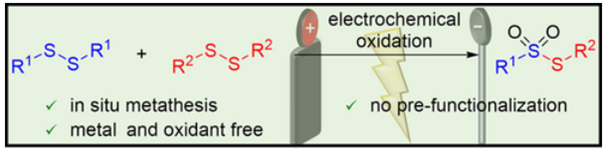
Synthesis of Symmetrical and Unsymmetrical Thiosulfonates from Disulfides via Electrochemical Induced Disulfide Bond Metathesis and Site-Selective Oxidation.
176. J. Fährmann, G. Hilt, Angew. Chem. 2021, 133, 20476-20480; DOI: 10.1002/ange.202107148, Angew. Chem. Int. Ed. 2021, 60, 20313-20317; DOI: 10.1002/anie.202107148
Alternating Current Electrolysis as Efficient Tool for the Direct Electrochemical Oxidation of Hydroxamic Acids for Acyl Nitroso Diels-Alder Reactions.
175. L. Li, G. Hilt, Chem. Eur. J. 2021, 27, 11221-11225; DOI: 10.1002/chem.202101259
Indium Tribromide-catalysed Transfer-Hydrogenation - Expanding the Scope of the Hydrogenation and of the Regiodivergent DH or HD Addition to Alkenes.
174. J. Fährmann, G. Hilt, Chem. Eur. J. 2021, 27, 11141-11149; DOI: 10.1002/chem.202101023
Electrochemical Synthesis of Organic Polysulfides from Disulfides via Sulfur Insertion from S8 and an Unexpected Solvent Effect on the Product Distribution.
173. J. Strehl, M. L. Abraham, G. Hilt, Angew. Chem. 2021, 133, 10084-10088; DOI: 10.1002/ange.202016413, Angew. Chem. Int. Ed. 2021, 60, 9996-10000; DOI: 10.1002/anie.202016413
Linear Paired Electrolysis - Realizing 200% Current Efficiency for Stochiometric Transformations - The Electrochemical Bromination of Alkenes.
172. S. M. Weber, G. Hilt, Front. Chem. 2021, 9, 635826; DOI: 10.3389/fchem.2021.635826
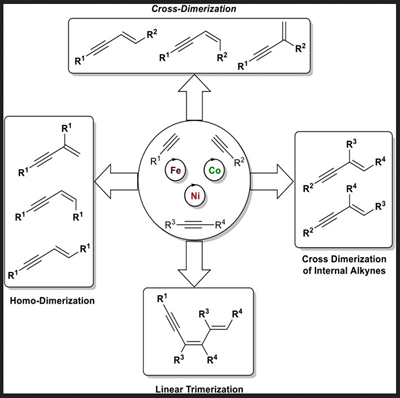
Late 3d Metal-Catalyzed (Cross)-Dimerization of Terminal and Internal Alkynes.
171. B. P. Klein, L. Ruppenthal, S. J. Hall, L. E. Sattler, S. M. Weber, J. Herritsch, A. Jaegermann, R. Maurer, G. Hilt, M. Gottfried, ChemPhysChem. 2021, 22, 1065-1073; DOI: 10.1002/cphc.202100222
Topology Effects in Molecular Organic Electronic Materials: Pyrene and Azupyrene.
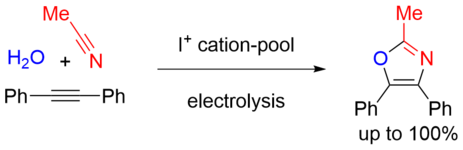
170. L. E. Sattler, G. Hilt, Chem. Eur. J. 2021, 27, 605-608; DOI: 10.1002/chem.202004140
Iodonium Cation-Pool Electrolysis for the Three-Component Synthesis of 1,3-Oxazoles.
2020
169. C. Kohlmeyer, A. Schäfer, P. H. Huy, G. Hilt, ACS Catal. 2020, 10, 11567-11577. DOI: 10.1021/acs.catal0c03348
Formamide Catalyzed Nucleophilic Substitutions: Mechanistic Insight and Rationalization of Catalytic Activity.
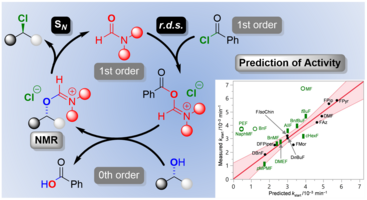
168. J. Strehl, G. Hilt, Org. Lett. 2020, 22, 5968-5972. DOI: 10.1021/acs.orglett.0c02068
Electrochemical, Iodine-Mediated a-CH Amination of Ketones by Umpolung of Silyl Enol Ethers.
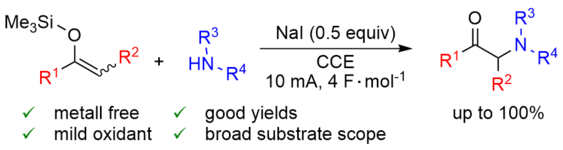
167. C. K. Krug, M. Zugermeier, J. Kuttner, M. Schmid, G. Hilt, J. M. Gottfried, J. Phys. Chem. 2020, 124, 15928-15934. DOI: 10.1021/acs.jpcc.0c03601
Polymorphism at the Metal/Organic Interface: Hybrid Phase with Alternating Coplanar and Vertical Adsorption Geometry.
166. L. E. Sattler, G. Hilt, J. Org. Chem. 2020, 85, 7595-7602. DOI: 10.1021/acs.joc.0c00776
Allylic Oxidation of Ester-substituted 1,4-Dienes.

165. S. M. Weber, J. Queder, G. Hilt, Chem. Eur. J. 2020, 26, 12129-12133. DOI: 10.1002/chem202001697
Ligand-Controlled Diastereoselective Cobalt-Catalysed Hydroalkynylation of Terminal Alkynes to E- or Z-1,3-Enynes.
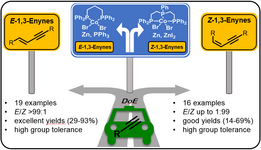
164. E. Babaoglu, G. Hilt, Chem. Eur. J. 2020, 26, 8879-8884. DOI: 10.1002/chem202001465
Electrochemical Iodine-Mediated Oxidation of Enamino-Esters to 2H-Azirine-2-Carboxylates supported by Design of Experiments.
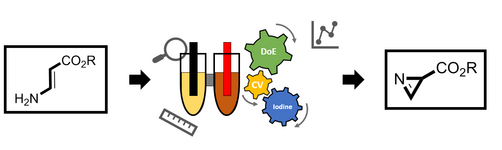
163. L. Li, G. Hilt, Org. Lett. 2020, 22, 1628-1632. DOI: 10.1021/acs.orglett.0c00213
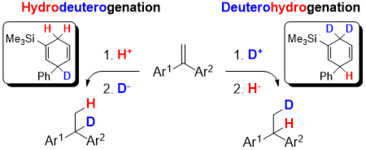
Regiodivergent DH or HD Addition to Alkenes: Deuterohydrogenation vs. Hydrodeuterogenation.
162. M. Hapke, G. Hilt, Cobalt-Catalysis in Organic Synthesis: Methods and Reactions, 2020, Wiley-VCH, DOI: 10.1002/9783527814855 ISBN: 9783527344505
161. L. E. Sattler, C. J. Otten, G. Hilt, Chem. Eur. J. 2020, 26, 3129-3136. DOI: 10.1002/chem.201904948
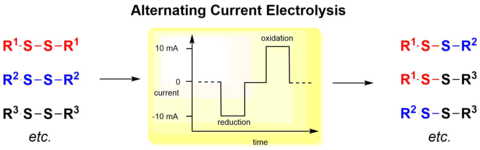
Alternating Current Electrolysis for the Electrocatalytic Synthesis of Mixed Disulfide via Sulfur-Sulfur Bond Metathesis towards Dynmic Disulfide Libraries.
160. J. Strehl, C. Kahrs, T. Müller, G. Hilt, J. Christoffers, Chem. Eur. J. 2020, 26, 3222-3225. DOI: 10.1002/chem.201905570
Electrochemical Induced Ring Transformation of Cyclic α-(ortho-Iodophenyl)-β-oxoesters.
159. G. Hilt, ChemElectroChem 2020, 7, 395-405. DOI:10.1002/celc.201901799
Basic Strategies and Types of Applications in Organic Electrochemistry.
2019
92a. F. Pünner, A. Schmidt, G. Hilt, Angew. Chem. 2019, 131, 17261-17262. DOI:10.1002/ange.201902504
Correction: Up the Hill: Selektive Doppelbindungsisomerisierung von terminalen 1,3-Dienen zu Z-1,3-Dienen oder 2Z,4E-Dienen.
158. F. Weber, G. Hilt, Cobalt-Catalyzed Hydrogenations, in "Homogeneous Hydrogenation with Non‐Precious Catalysts", F. Teichert (Ed.), 2020, Wiley-VCH, S. 39-61. DOI: 10.1002/9783527814237.ch2 ISBN: 9783527344390
157. Q. Fan, D. Martin-Jimenez, D. Ebeling, C. K. Krug, L. Brechmann, C. Kohlmeyer, G. Hilt, W. Hieringer, A. Schirmeisen, J. M. Gottfried, J. Am. Chem. Soc. 2019, 141, 17713-17720. DOI: 10.1021/jacs.9b08060
Nanoribbons with Nonalternant Topology from Fusion of Polyazulene: Carbon Allotropes beyond Graphene.
156. J. Strehl, G. Hilt, Org. Lett. 2019, 21, 5259-5263. DOI: 10.1021/acs.orglett.9b01866
Electrochemical, Manganese-Assisted Carbon-Carbon Bond Formation between β-Keto Esters ans Silyl Enol Ethers.
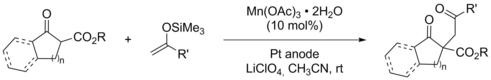
155. S. M. Weber, G. Hilt, Org. Lett. 2019, 21, 4106-4110. DOI: 10.1021/acs.orglett.9b01281
Chemoselective Cobalt(I)-Catalyzed Cyclotrimerization of (Un)Symmetrical 1,3-Butadiynes for the Synthesis of 1,2,4-Regioisomers.
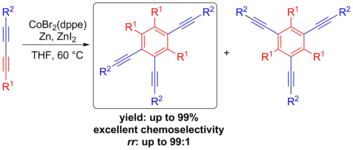
2018
154. R. Möckel, E. Babaoglu, G. Hilt, Chem. Eur, J. 2018, 24, 15781-15785. DOI: 10.1002/chem.201804152
Iodine(III)‐mediated Electrochemical Trifluoroethoxylactonisation ‐ Rational Reaction Optimisation and Prediction of Mediator Activity.
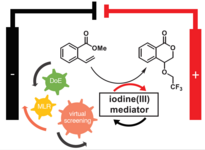
153. Q. T. Fan, S. Werner, J. Tschakert, D. Ebeling, A. Schirmeisen, G. Hilt, W. Hieringer, J.M. Gottfried, J. Am. Chem. Soc. 2018, 140, 7526-7532. DOI: 10.1021/jacs.8b01658.
Precise Mono-Selective Aromatic C-H Bond Activation by Chemisorption of Meta-Aryne on a Metal Surface.
152. Q. T. Fan, L. Liu, J. Dai, T. Wang, H. Ju, J. Zhao, J. Kuttner, G. Hilt, J. M. Gottfried, J. F. Zhu, ACS Nano 2018, 12, 2267-2274. DOI: 10.1021/acsnano.7b06787.
Surface Adatom Mediated Structural Transformation in Bromoarene Monolayers: Precursor Phases in Surface Ullmann Reaction.
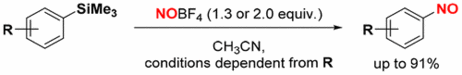
151. C. Kohlmeyer, M. Klüppel, G. Hilt, J. Org. Chem. 2018, 83, 3915-3920. DOI: 10.1021/acs.joc.8b00262
Synthesis of Nitrosobenzene Derivatives via Nitrosodesilylation Reaction.
150. M. Ballmann, F. Weber, L. E. Sattler, G. Hilt, Synthesis 2018, 50, 1711-1720. DOI: 10.1055/s-0036-1591878
Synthesis of non conjugated Trienes via in situ Hydrovinylation/Wittig Olefination of unsaturated Phosphonium Salts.

149. R. Möckel, J. Hille, E. Winterling, S. Weidemüller, T. M. Faber, G. Hilt, Angew. Chem. 2018, 130, 450-454. DOI: 10.1002/ange.201711293; Angew. Chem. Int. Ed. 2018, 57, 442-445. DOI: 10.1002/anie.201711293.
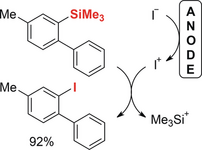
Elektrochemische Synthese von Aryliodiden durch anodische Iododesilylierung.
2017
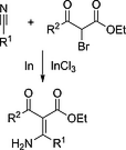
148. L. Li, E. Babaoglu, K. Harms, G. Hilt, Eur. J. Org. Chem. 2017, 4543-4547. DOI: 10.1002/ejoc.201700868
Expanding Blaise-Type Reactions towards Indium-Mediated Transformations of α-Bromo-β-keto Esters with Nitriles.
147. M. Chen, J. Shang, Y. Wang, K. Wu, J. Kuttner, G. Hilt, W. Hieringer, J. M. Gottfried, ACS Nano 2017, 11, 134-143. DOI: 10.1021/acsnano.6b05709
On-Suface Synthesis and Characterization of Honeycombene Oligophenylene Macrocycles.
146. P. Röse, G. Hilt, Adv. Synth. Catal. 2017, 359, 1359-1372.
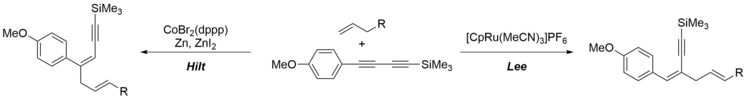
145. S. M. Weber, G. Hilt, Org. Lett. 2017, 19, 564-567. DOI: 10.1021/acs.orglett.6b03729
Control of the Regioselectivity in Cobalt- versus Ruthenium-Catalyzed Alder-ene Reactions of Unsymmetrical 1,3-Diynes.
144. Q. Fan, T. Wang, J. Dai, J. Kuttner, G. Hilt, J. M. Gottfried, J. Zhu, ACS Nano 2017, 11, 5070-5079. DOI: 10.1021/acsnano.7b01870
On-Surface Pseudo-High Dilution Synthesis of Macrocycles: Principle and Mechanism.
143. F. Weber, P. S. Steinlandt, M. Ballmann, G. Hilt, Synthesis 2017, 49, 440-450. DOI: 10.1055/s-0036-1588340
Structure dependent Nickel-catalysed Transposition of N-Allylamides to E- or Z-Enamides.
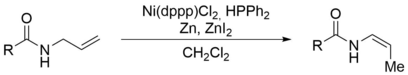
2016
142. A. Nödling, R. Möckel, R. Tonner, G. Hilt, Chem. Eur. J. 2016, 22, 13171-13180. DOI: 10.1002/chem.201602394
Lewis acids as activators in CBS-catalysed Diels-Alder reactions - distortion induced Lewis acidity enhancement of SnCl4.
141. E. Babaoglu, K. Harms, G. Hilt, Synlett 2016, 27, 1820-1823. DOI: 10.1055/s-0035-1561973
Indium-Mediated Blaise-Type Reaction of Bromomalonates with Nitriles and Isocyanates.
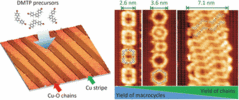
140. Q. Fan, J. Dai, T. Wang, J. Kuttner, G. Hilt, J. M. Gottfried, J. Zhu, ACS Nano 2016, 10, 3747-3754. DOI:10.1021/acsnano.6b00366
Confined Synthesis of Organometallic Chains and Macrocycles by Cu-O Surface Templating.
139. J. Dai, Q. Fan, T. Wang, J. Kuttner, G. Hilt, J. M. Gottfried, J. Zhu, Physical Chemistry Chemical Physics 2016, 18, 20627-20634. DOI: 10.1039/C6CP03551E
The role of the substrate structure in the on-surface synthesis of organometallic and covalent oligophenylene chains.
138. F. Weber, M. Ballmann, C. Kohlmeyer, G. Hilt, Org. Lett. 2016, 18, 548-551. DOI:10.1021/acs.orglett.5b03585
Nickel-Catalyzed Double Bond Transposition of Alkenyl-Boronates for in situ syn-Selective Allylboration Reactions.
137. P. Röse, G. Hilt, Synthesis 2016, 48, 463-492. DOI:10.1021/acs.joc.5b01198
Cobalt-catalyzed Bond Formation Reactions; Part 2.
2015
136. P. Röse, C. C. M. Garcia, F. Pünner, K. Harms, G. Hilt, J. Org. Chem. 2015, 80, 7311-7316. DOI: 10.1021/acs.joc.5b01198
Cobalt-catalyzed Cross-Benzannulation of Conjugated Enynes and Diynes.
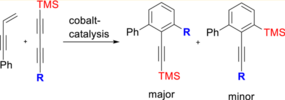
135. F. Weber, A. Schmidt, P. Röse, M. Fischer, O. Burghaus, G. Hilt, Org. Lett. 2015, 17, 2952-2955. DOI:10.1021/acs.orglett.5b01230
Double Bond Isomerization - Highly Reactive Nickel Catalyst Applied in the Synthesis of the Pheromone (9Z,12Z)-Tetradeca-9,12-dienyl Acetate.
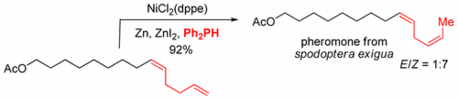
134. C. Wang, Y. Zheng, H. Huo, P. Röse, L. Zhang, K. Harms, G. Hilt, E. Meggers, Chem. Eur. J. 2015, 21, 7355-7359. DOI: 10.1002/chem.201500998
Merger of Visible Light Induced Oxidation and Enantioselective Alkylation with a Chiral Iridium Catalyst.
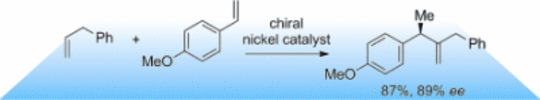
133. G. Hilt, ChemCatChem. 2015, 7, 1639-1641. DOI: 10.1002/cctc.201500177
Asymmetric Nickel-Catalysed Cross-Hydrovinylation of Two Terminal Alkenes.
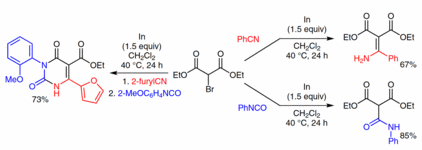
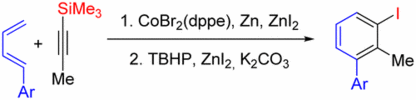
132. R. Möckel, G. Hilt, Org. Lett. 2015, 17, 1644-1647. DOI: 10.1021/acs.orglett.5b00306
Synthesis of Polysubstituted Iodobenzene Derivatives from Alkynylsilanes and 1,3-Dienes via Diels-Alder / Oxidation / Iodination Reaction Sequence.
131. J. Kuttner, G. Hilt, Synthesis 2015, 47, 1170-1180. DOI: 10.1055/s-0034-1380148
Synthesis of Acyclic Polycarbonyl Compounds via Ozonolysis of 1,4-Cyclohexadienes.
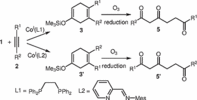
130. P. Röse, S. Emge, J.-i. Yoshida, G. Hilt, Beilstein J. Org. Chem. 2015, 11, 174-183. DOI: 10.3762/bjoc.11.18
Electrochemical Selenium- and Iodonium-Initiated Cyclisation of Hydroxy-functionalised 1,4-Dienes.
129. J. Shang, Y. Wang, M. Chen, J. Dai, X. Zhou, J. Kuttner, G. Hilt, J. M. Gottfried, K. Wu, Nature Chem. 2015, 7, 389-393. DOI: 10.1038/nchem.2211
Assembling Molecular Sierpinski Triangles Fractals.
128. A. Schmidt, A. Nödling, G. Hilt, Angew. Chem. 2015, 127, 814-818. DOI: 10.1002/ange.201409902; Angew. Chem. Int. Ed. 2015, 54, 801-804. DOI: 10.1002/anie.201409902
An Alternative Mechanism for the Cobalt-Catalyzed Isomerization of Terminal Alkenes to (Z)-2-Alkenes.
2014
127. L. Kersten, G. Hilt, J. Org. Chem. 2014, 79, 11661-11673. DOI: 10.1021/jo502308d
Synthesis of Tri-, Tetra- and Pentacarbonyl Derivatives via Ozonolysis of 1,4-Dienes and Cyclisation to Polyaromatic Systems.
126. H. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt, E. Meggers, Nature 2014, 515, 100-103. DOI: 10.1038/nature13892
Asymmetric photoredox transition-metal catalysis activated by visible light.
125. A. Nödling, G. Jakab, P. R. Schreiner, G. Hilt, Eur. J. Org. Chem. 2014, 6394-6398. DOI:10.1002/ejoc.201402871
31P NMR Spectroscopically Quantified Hydrogen-Bonding Strength of Thioureas and Their Activity in Diels-Alder Reactions.
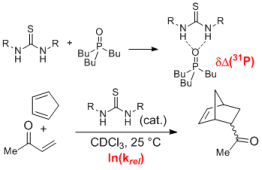
124. J. Kuttner, G. Hilt, Macromolecules 2014, 47, 5532-5541. DOI:10.1021/ma5012446
Regiodivergent Cobalt-Catalysed Diels-Alder Reactions for the Synthesis of Bifunctional Building Blocks and their Suzuki-Cross-Coupling Polymerisations.
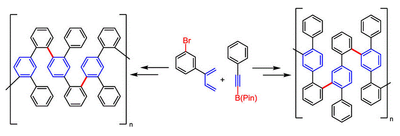
123. G. Hilt, ChemCatChem 2014, 6, 2484-2485. DOI: 10.1002/cctc.201402341
Double Bond Isomerisation and Migration - New Playgrounds for Transition Metal-Catalysis.
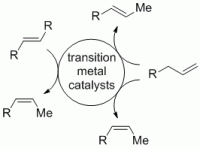
122. A. Schmidt, E. Maiterth, G. Hilt, Synthesis 2014, 46, 2040-2044. DOI: 10.1055/s-0034-1378375
Cobalt-Catalysed Transformations of 1,3,5-Hexatrienes on a Large Scale.
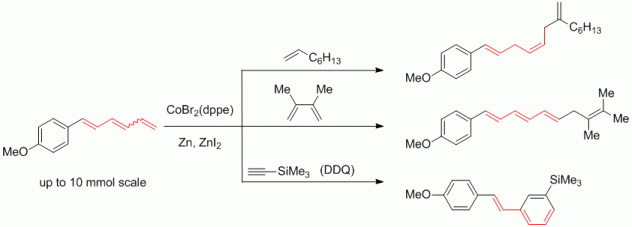
121. P. Susnik, G. Hilt, Organometallics 2014, 33, 5907-5910. DOI: 10.1021/om500292t
Homoallylpinacolboronic Ester as Alkene Component in Cobalt-Catalyzed Alder-ene Reactions.
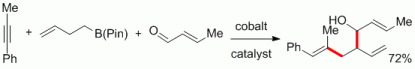
120. F. Pünner, G. Hilt, Chem. Commun. 2014, 50, 7310-7313. DOI:10.1039/C4CC03348E
Zinc-mediated CH-activation of tetrahydrofuran under mild conditions for the regioselective addition to aryl-propiolates.
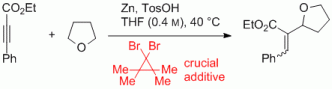
119. Q. Fan, C. Wang, L. Liu, J. Zhao, J. Kuttner, G. Hilt, J. M. Gottfried, J. Phys. Chem. 2014, 118, 13018-13025. DOI:10.1021/jp5037475
Covalent, Organometallic and Halogen Bonded Nanomeshes from Tetrabromo-Terphenyl by Surface-Assisted Synthesis on Cu(111).

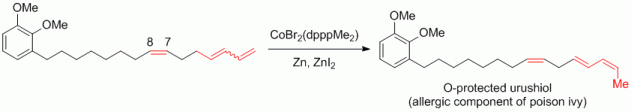
118. A. Schmidt, G. Hilt, Chem. Asian J. 2014, 9, 2407-2410. DOI: 10.1002/asia.201402323
Unprecedented Cobalt-Catalysed Isomerisation Reaction to Single Skipped 2,4,7-Trienes Applied in the Synthesis of Urushiol.
117. G. Hilt, Chem. Rec. 2014, 14, 386-396. DOI: 10.1002/tcr.201400001
1,4-Cyclohexadienes - Easy Access to a Versatile Building Block via Transition Metal-Catalysed Diels-Alder Reactions.
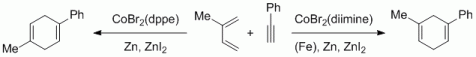
116. A. R. Nödling, K. Müther, V. H. G. Rohde, G. Hilt, M. Oestreich, Organometallics 2014, 33, 302-308. DOI: 10.1021/om401040y
Ferrocene-Stabilized Silicon Cations as Catalysts for Diels-Alder Reactions: Attempted Experimental Quantification of Lewis Acidity and ReactIR Kinetic Analysis.
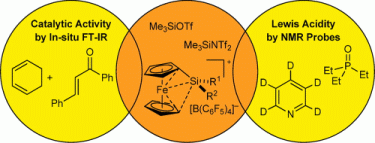
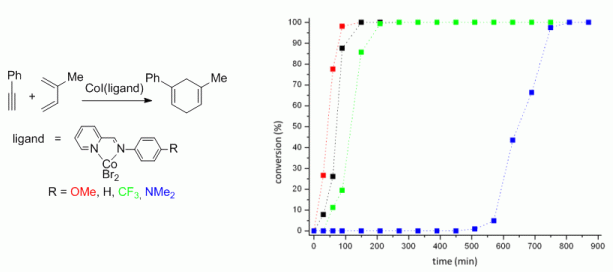
115. G. Hilt, J. Janikowski, M. Schwarzer, O. Burghaus, D. Sakow, M. Bröring, M. Drüschler, B. Huber, B. Roling, G. Frenking, J. Organomet. Chem. 2014, 749, 219-223. DOI: 10.1016/j.bbr.2011.03.031
Studies of Electronic Effects of Modified Pyridine-Imine Ligand Utilised in Cobalt-catalysed meta-selective Diels-Alder Reactions.
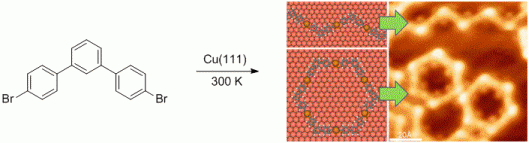
114. Q. Fan, C. Wang, Y. Han, J. Zhu, J. Kuttner, G. Hilt, J. M. Gottfried, ACS Nano 2014, 8, 709-718. DOI: 10.1021/nn405370s
Surface-assisted Formation, Assembly and Dynamics of Planar Organometalic Macrocycles and Zigzag Shaped Polymer Chains with C-Cu-C Bonds.
113. P. Raster, A. Schmidt, M. Rambow, N. Kuzmanovic, B. König, G. Hilt, Chem. Commun. 2014, 50, 1864-1866. DOI: 10.1039/C3CC48487D
Immobilisation of Photoswitchable Diarylhexenes Synthesised via Cobalt-Mediated Diels-Alder Reaction.
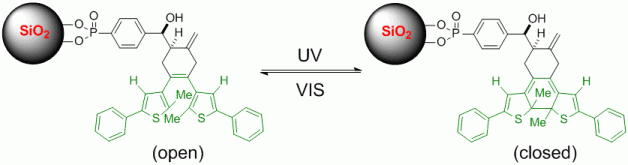
112. A. Miersch, K. Harms, G. Hilt, Chem. Commun. 2014, 50, 542-544. DOI: 10.1039/C3CC46788K
Zinc-Mediated Addition of Diethyl Bromomalonate to Alkynes for the Initiation of Multi-Component Reactions toward Polysubstituted Pyranones and Tetracarbonyl Derivatives.
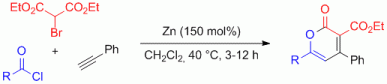
2013
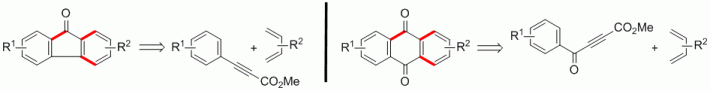
111. F. Pünner, J. Schieven, G. Hilt, Org. Lett. 2013, 15, 4888-4891. DOI: 10.1021/ol4023276
Synthesis of Fluorenone and Anthraquinone Derivatives from Aryl- and Aroyl-substituted Propiolates.
110. A. Miersch, C. Kohlmeyer, G. Hilt, Synthesis 2013, 3228-3232. DOI: 10.1055/s-0033-1339616
Zinc-Mediated Regiodiverse Synthesis of Vinyl Bromide Derivatives and their in situ Palladium-catalysed Cross-Coupling Reactions.
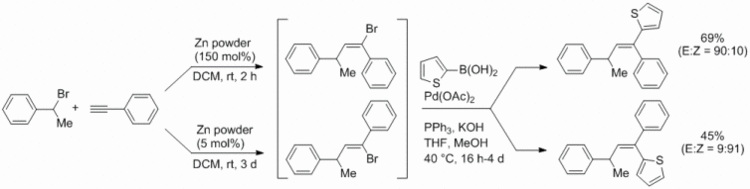
109. F. Pünner, G. Hilt, Eur. J. Org. Chem. 2013, 5580-5584. DOI: 10.1002/ejoc.201300726
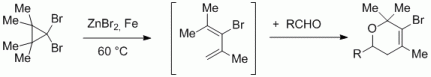
Zinc/Iron-mediated Ring-Opening of Dibromocyclopropanes for in situ Diels-Alder Reactions with Electron-deficient Aldehydes and Imines.
108. L. Fiebig, J. Kuttner, G. Hilt, M. Schwarzer, G. Frenking, H.-G. Schmalz, M. Schäfer, J. Org. Chem. 2013, 78, 10485-10493. DOI:10.1021/jo402001g
Cobalt Catalysis in the Gas Phase: Experimental Characterisation of Cobalt(I) Complexes as Intermediates in Regioselective Diels-Alder Reactions.
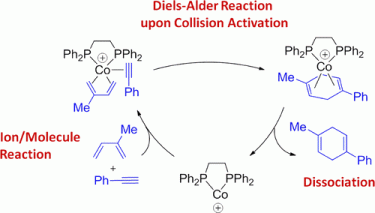
107. A. Schmidt, G. Hilt, Org. Lett. 2013, 15, 2708-2711. DOI:10.1021/ol401015e
Scope and Limitations of 1,3,5-Hexatriene Derivatives in Regioselective Cobalt-Catalyzed Reactions.
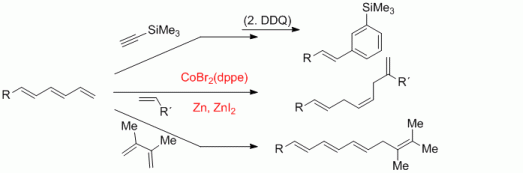
106. P. Röse, F. Pünner, G. Hilt, K. Harms, Synlett 2013, 1101-1104. DOI:10.1055/s-0033-1338384
Efficient Synthesis of 2-Pyridylenynes and Application in Cobalt-Catalysed Benzannulation Reactions.
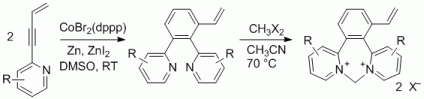
105. Q. Fan, J. Zhu, J. Kuttner, G. Hilt, J. M. Gottfried, Angew. Chem. 2013, 125, 4766-4770; Angew. Chem. Int. Ed. 2013, 52, 4668-4672. DOI: 10.1002/anie.201300610
Surface-Assisted Organic Synthesis of Hyperbenzene Nanotroughs.
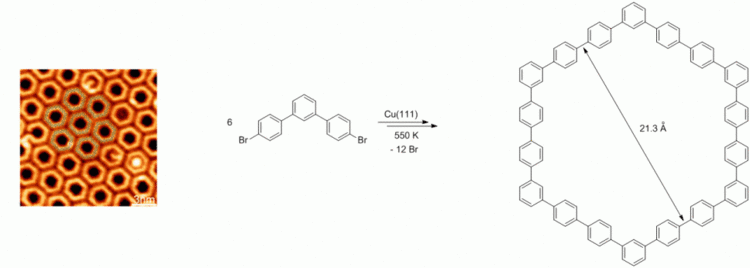
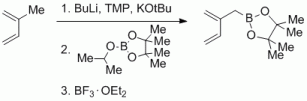
104. G. Hilt, EROS-Reagent 4,4,5,5-Tetramethyl-2-(2-methylene-3-buten-1-yl)-1,3,2-dioxaborolane. DOI: 10.1002/047084289X.rn01618
96a. Addition to 96.: M. Arndt, G Hilt, A. F. Khlebnikov, S. I. Kozhushkov, A. de Meijere, Eur. J. Org. Chem. 2013, 1171-1172. DOI: 10.1002/ejoc.201200105
Diels-Alder Reactions for the Construction of Cyclopropylarenes.
2012
103. M. Arndt, M. Dindaroğlu, H.-G. Schmalz, G. Hilt, Synthesis 2012, 44, 3534-3542. DOI: 10.1055/s-0030-1258408
Ligand Control of the Cobalt-Catalysed 1,4-Hydrovinylation Reaction.
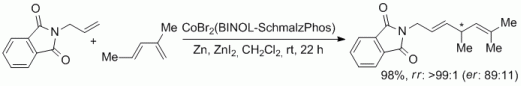
102. F. Erver, J. R. Kuttner, G. Hilt, J. Org. Chem. 2012, 77, 8375-8385. DOI: 10.1021/jo301028b
Multidirectional Cobalt-Catalyzed Diels-Alder / Hydrovinylation Sequences.
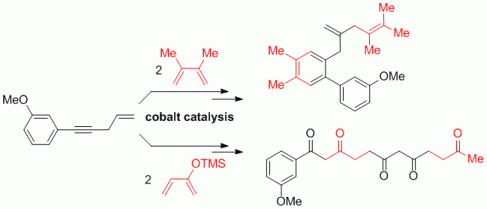
101. A. Miersch, G. Hilt, Chem. Eur. J. 2012, 18, 9798-9801. DOI: 10.1002/chem.201201385
Stereodivergent Zinc-Mediated Three-Component Synthesis of Tri- and Tetrasubstituted Alkenes.
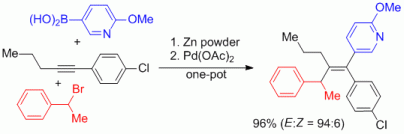
100. F. Erver, G. Hilt, J. Org. Chem. 2012, 77, 5216-5219. DOI: 10.1021/jo3007896
Cobalt- versus Ruthenium-catalyzed Alder-ene Reaction for the Synthesis of Credneramides A and B.
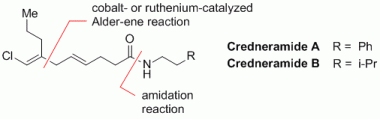
99. F. Erver, G. Hilt, Org. Lett. 2012, 14, 1884-1887. DOI: 10.1021/ol300504f
Double- and Triple-Cobalt-Catalysis in Multi-Component Reactions.
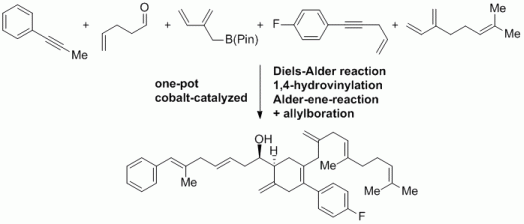
98. G. Hilt, Eur. J. Org. Chem. 2012, 4441-4451. DOI: 10.1002/ejoc.201200212
The Hydrovinylation Reactions - Atom-Economic Transformations with Steadily Increasing Synthetic Potential.
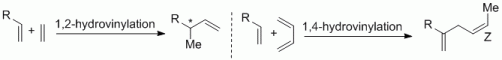
97. G. Hilt, F. Pünner, Diels-Alder Reactions, in Transition-Metal-Mediated Aromatic Ring Construction, K. Tanaka, (Ed.), Wiley, 2012, 342. ISBN:978-1-118-14892-1
96. M. Arndt, G. Hilt, A. F. Khlebnikov, S. I. Kozhushkov, A. de Meijere, Eur. J. Org. Chem. 2012, 3112-3121. DOI: 10.1002/ejoc.201200105
Diels-Alder Reactions for the Construction of Cyclopropylarenes.
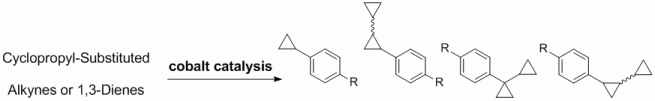
95. F. Pünner, G Hilt, Chem. Commun. 2012, 3617-3619. DOI: 10.1039/C2CC30777D
Regioselective Solvent-dependent Benzannulation of Conjugated Enynes.
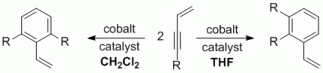
94. J. R. Kuttner, S. Warratz, G Hilt, Synthesis 2012, 44, 1293-1303. DOI: 10.1055/s-0031-1289752
Straightforward Synthesis of Non-Conjugated Cyclohex-3-enones and Conjugated 4-Methylene-cyclohex-2-enone Derivatives.
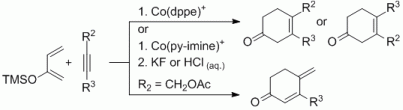
93. L. Kersten, G. Hilt, Adv. Synth. Catal. 2012, 354, 863-869. DOI: 10.1002/adsc.201100800
Regioselective Cobalt-Catalysed Hydrovinylation for the Synthesis of non-conjugated Enones and 1,4-Diketones.
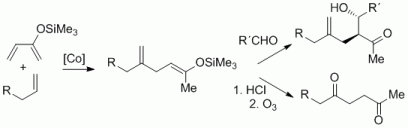
92. F. Pünner, A. Schmidt, G. Hilt, Angew. Chem. 2012, 124, 1296-1299, DOI: 10.1002/ange.201107512 ; Angew. Chem. Int. Ed. 2012, 51, 1270-1273. DOI: 10.1002/anie.201107512
Up the Hill: Selective Double Bond Isomerisation of Terminal 1,3-Dienes towards Z-1,3-Dienes or 2Z,4E-2,4-Dienes.
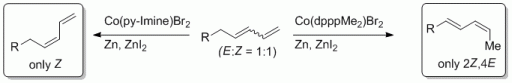
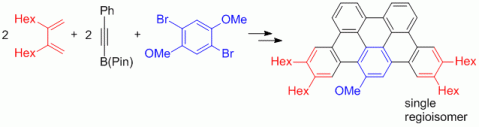
91. M. Danz, R. Tonner, G. Hilt, Chem. Commun. 2012, 377-379. DOI: 10.1039/C1CC15980A
Understanding the Regioselectivity in Scholl Reactions for the Synthesis of Oligoarenes.
90. G. Kiefer, J. Ruiz, E. Solari, G. Hilt, K. Severin, Eur. J. Org. Chem. 2012, 93-98. DOI: 10.1002/ejoc.201101263
Ruthenium-catalyzed Sequential Enyne Cross-Metathesis/ATRA Reactions.