Publications
18. Niko Kruse, Kerry Hazeldine, Martin Hedevang, Celina Groothuis, Duy Le, Talat S. Rahman, Jeppe V. Lauritsen, Lars Mohrhusen*: Monolayer TiS2 Nanosheets on Au(111)–Structural Characterization and Effect of Edge Stability for Shape Control
accepted in Small: DOI:10.1002/smll.202506023
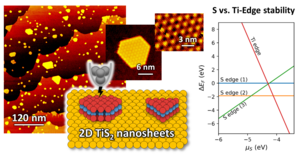
Transition metal dichalcogenides are promising alternatives to noble metal catalysts, e.g., for (photo-)activation of greenhouse gases or hydrogenations. Herein, a direct synthetic route for 2D TiS2 nanosheets on Au(111) by titanium deposition in the presence of a mild, organic, non-oxidizing sulfur source is presented. High-resolution scanning tunneling microscopy (STM) is used to gain atomic-level insights into the TiS2 nanosheet morphology. In contrast to the literature, this protocol gains mostly hexagonal and truncated triangular nanosheets with an increased edge contrast in STM, analog to metallic edge states in MoS2. Synchrotron-based photoelectron spectroscopy allows insights into compositional details, specifically to distinguish different S sites on the TiS2 sheets and other S species on the sample. Further, a minimum size is identified (9 S atoms side length), which underlines the importance of moiré reconstructions for stress relief. The TiS2 sheets coexist with [Au]Ti1S3 clusters, in which a single gold atom is alloyed into the surface and capped by three S atoms. Together with the finding of a critical sheet size, this points toward on-surface Ostwald ripening as a relevant process in the sheet formation. Ab-initio calculations (density functional theory) underscore that the chemical potential of S is an essential descriptor to maintain shape control.
17. Lars Mohrhusen, Shengjie Zhang, Matthew M. Montemore and Robert J. Madix: Modifying the reactivity of single Pd sites in a trimetallic Sn-Pd-Ag surface alloy: Tuning CO binding strength
Small 20, 48, 2405715 (2024).
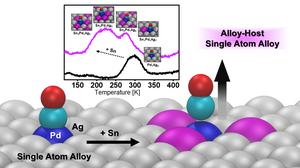
Improving control over active-site reactivity is a grand challenge in catalysis. Single-atom alloys (SAAs) consisting of a reactive component doped as single atoms into a more inert host metal feature localized and well-defined active sites, but fine tuning their properties is challenging. Here, we develop a framework for tuning single-atom site reactivity by alloying in an additional inert metal, which we term an alloy-host SAA. Specifically, we created about 5% Pd single-atom sites in a Pd33Ag67(111) single-crystal surface, and then identified Sn based on computational screening as a suitable third metal to introduce. Subsequent experimental studies show that introducing Sn indeed modifies the electronic structure and chemical reactivity (measured by CO desorption energies) of the Pd sites. The modifications to both the electronic structure and the CO adsorption energies are in close agreement with our calculations. These results indicate that the use of an alloy host environment to modify the reactivity of single-atom sites could allow fine-tuning of catalytic performance and boost resistance against strong-binding adsorbates such as CO.
16. Lars Mohrhusen, Tobias Egle, Jennifer D Lee, Cynthia M Friend, Robert J Madix: Creation of Well-Defined Pd Surface Sites on Single Crystal Pd33Ag67: From Ensembles to Single Atoms
J. Phys. Chem. C 126, 48, 20332-20342 (2022).
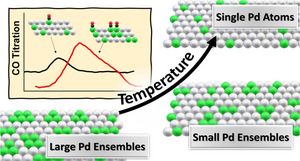
The composition and distribution of Pd atoms on the surface were controlled by thermal treatments following sputtering of the surface of PdAg single crystal of composition Pd33Ag67 at 100 K. The Pd distribution at the (111) surface was varied from small ensembles to single atoms by thermal annealing between 400 and 820 K. On Pd ensembles, D2 dissociates readily, populating the surface with two types of atomic D/H. D2 (H2) desorbs around 295 K (β-state) from pure Pd sites, while it desorbs from bimetallic or pure silver sites desorbs around 185-230 K (γ-state). In contrast, single Pd sites did not dissociate D2 or H2 under the conditions used herein. In the annealing process rapid segregation of Pd from the topmost surface into the near subsurface occurs with annealing within minutes, and a subsequent, much slower diffusion into the bulk occurs on the time scale of hours. Furthermore, subsurface Pd can be employed for fine adjustment of the chemical bonding of adsorbates to these surfaces. This study establishes a method for tuning the compositional and configurational surface properties of well-defined substitutional bimetallic bulk alloys.
15. Lars Mohrhusen* and Katharina Al-Shamery: Conversion of Alcohols on Stoichiometric and Reduced Rutile TiO2 (110): Point Defects Meet Bifunctionality in Oxide (Photo-)Chemistry
Catalysis Letters 153, 321-337 (2023).

Oxidic (photo-)catalysts have the potential to play an important role to efficiently implement sustainable feedstocks and green energy sources into future energy technologies. They may be used not only for solar energy harvesting, but also for hydrogen production or being essential for the fabrication of fine chemicals. Therefore, it is crucial to develop a detailed understanding of how the atomistic environment of the catalyst can be designed in order to promote distinct reaction pathways to influence the final product distribution of chemical reactions. In this perspective article, we survey the surface (photo-)chemistry of methanol on rutile TiO2 surfaces and hybrid catalysts based thereon. Especially the role of the surface bifunctionality by Lewis acidic and basic sites combined with the strong impact of point defects such as reduced titanium sites (mainly Ti3+ interstitials) shall be illuminated. It is shown how the selective activation of either O-H, C-H or C-O bonds in the methanol molecule can be used to tune not only the overall conversion, but to switch between oxidative and reductive routes in favor of either deoxygenation, partial oxidation or C-C coupling reactions. Especially the latter ones are of particular interest to introduce methanol from green sources such as biomass as a sustainable feedstock into already existing petrochemical technologies.
14. Lars Mohrhusen*, N.Brinkmann, K. Obermann, E.C. Atata, J.Kräuter, M.Osmic, M.Grebien, Katharina Al-Shamery: Thermal Stability of Ionic Compounds on Nanostructured Metal Catalysts: Conversion of Quaternary Ammonium to Amines on Gold Nanoparticles
J. Phys. Chem. C 126, 39, 16690-16701 (2022).
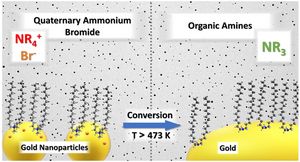
Quaternary ammonium halides are an important class of compounds because they are not only used as structural templates ororganic surface modifiers for inorganic minerals and other aluminosilicates such as zeolites, but are also well-known and widespread as phase-transfer catalysts and stabilizing agents for colloidal metal nanoparticle synthesis. Moreover, quaternary ammonium ions are frequently exploited as weakly coordinating cations in various ionic liquids. Thus, it is important to monitor, understand, and utilize thermal effects and limits of application. Herein, we report the thermal conversion of didodecyldimethylammonium bromide (DDAB) into organic amines on the surface of gold nanoparticles. In contrast to the pure compound, in which this decomposition appears as an endothermic process, the on-particle reaction is exothermic. This in situ modification of the ligand shell not only involves the essential step of halide removal through the gas phase but also turns out to be a valuable tool to produce uncovered metal sites and thus impacts the fabrication of nanostructured metal catalysts for ligand-directed chemistry.
13. Imke Maack, Milena Osmić, Lars Mohrhusen*, Pascal Buhani, Katharina Al-Shamery: Fitting A Square Peg Into A Round Hole: Shape Control in Phase Transfer of Cubic Gold Nanoparticles
ChemNanoMat 7, 658 - 671 (2021).
Although nanomaterials are widely involved in technological applications, common synthetic recipes for such colloids are restricted to special, optimized conditions, particularly for anisotropic shapes. Ligand exchange is frequently necessary for further functionalization. While such protocols are well established for spherical particles, it is more demanding to keep the corpus for thermodynamically less stable shapes. We highlight the temperature as one key for the formation of anisotropic gold nanoparticles, but also for ligand exchange protocols under shape retention or deliberate reshaping at the example of gold nanocubes. In the first part, the synthesis of CTAB capped gold nanocubes in aqueous solution is examined highlighting the narrow temperature window in which selective adsorption site blocking by bromide ions leads to the formation of gold nanocubes. While too low temperatures yield multiple particle shapes due to a low surface mobility, temperatures above the appropriate window result in more thermodynamically favored shapes. Furthermore, two protocols are presented for the exchange of the ammonium ligand CTAB by oleylamine as an organic amine including water removal from a slurrish water-amine paste through the gas phase. In turn, precise temperature control allows to either maintain the cubic shape or induce a reshaping process towards other, thermodynamically preferred shapes such as for example truncated octahedra.
12. Lars Mohrhusen*, Jessica Kräuter, Katharina Al-Shamery: Conversion of methanol on rutile TiO2(110) and tungsten oxide clusters: 2. the role of defects and electron transfer in bifunctional oxidic photocatalysts
Phys. Chem. Phys., 23, 12148-12157 (2021).
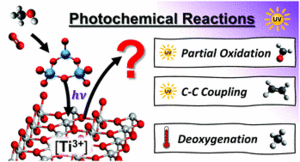
he photochemical conversion of organic compounds on tailored transition metal oxide surfaces by UV irradiation has found wide applications ranging from the production of chemicals to the degradation of organic pollutants e.g. in waste water treatment. Here, we present a systematic surface science-based study of the UV photoconversion of methanol on a rutile TiO2(110) surface. Under the used conditions, the dominant photoreaction is the photo-oxidation forming formaldehyde, that is drastically boosted by the presence of adsorbed oxygen as well as (sub-)surface defects such as oxygen vacancies and Ti3+ interstitials. Furthermore, a photostimulated and Ti3+ mediated C-C coupling was observed leading to the production of ethene. We have further deposited tungsten oxide clusters on the rutile surface and examined the impact on the methanol photochemistry. In this case, the C-C coupling can be suppressed. Surprisingly, especially for high Ti3+ contents the population of the photochemical pathway is quenched in favor of the population of the thermal reaction yielding more methane from the deoxygenation reaction. Thus, the common concept that long time charge separation is efficient by combining two photocatalysts with similar band gaps, but different work functions in order to enhance photochemical yields is apparently too naive for certain systems. We attribute the loss of photoproducts with tungsten oxide coadsorption to the "pinning" of Ti3+ centers and a related enhancement of electron density near the oxide clusters which makes a concomitant recombination of the photochemical relevant holes with the excess surface electrons more likely.
11. Lars Mohrhusen*, Katharina Al-Shamery: Conversion of methanol on rutile TiO2 (110) and tungsten oxide clusters: 1. population of defect-dependent thermal reaction pathways
Phys. Chem. Phys., 3, 12137-12147 (2021).

Tungsten oxide clusters deposited on rutile TiO2 (110) single crystals were used as a model system for heterogenous oxide-oxide bifunctional catalysts. The population of different thermal reaction routes in methanol conversion in the presence of preadsorbed oxygen was probed under UHV conditions. By temperature programmed reaction spectroscopy, we have identified three thermal reaction channels, namely the deoxygenation under formation of methane, the partial oxidation forming formaldehyde and the condensation route under desorption of ethane and dimethyl ether. The specific local reaction environment at the oxidic surface was found to be key for the population of the different reaction channels as exhibited by the introduction of Lewis acidic and basic sites (especially (WO3)n clusters) and available charge carriers such as Ti3+. Especially the amount of bulk Ti3+ interstitials, that can partially transfer charge towards the tungsten oxide clusters at the TiO2 surface, was found to be a key parameter that enables a relatively high methanol conversion in thermal reactions. It turned out that the deoxygenation is by far the most dominant reaction followed by the partial oxidation. The condensation is observed only in low amounts under special conditions, but is an interesting example for reactivity at defect sites.
10. Lars Mohrhusen*, Luca Gerhards, Daniel Hirsch, Thorsten Klüner, Katharina Al-Shamery: Multidentate Interaction of Methylamine with Rutile TiO2 (110)
J. Phys. Chem. C 125, 22, 11975-11986 (2021).
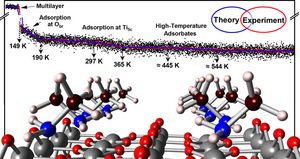
Aiming for a comprehensive expertise of the adsorption and activation of small organic molecules on transition metal oxides, the chemistry of methylamine on rutile TiO2 (110) is examined combining surface science experiments with first-principles calculations. Besides the physisorption of a methylamine multilayer, three types of methylamine adsorbates were ascertained: adsorption via hydrogen bonding toward bridging oxygen atoms, adsorption of methylamine at one individual Ti5c site, and an adsorbate occupying two Ti5c sites in an unprecedented multidentate bonding geometry. In all three adsorbate classes, multiple interactions with the TiO2 substrate as well as intermolecular dispersion forces are identified to be crucial to explain the molecule-surface interaction and concomitant adsorption geometries. While the Ti-N bond surely makes the largest contribution to the molecules' adsorption energy, smaller forces such as hydrogen bonds between NH-O and CH-O moieties are shown to be the central key for understanding the chemistry of methylamine on titania surfaces. Comparing different adsorption geometries, these multidentate adsorption motifs can boost the thermal stability in relation to ordinary adsorption, in the case of the adsorbate occupying two Ti5c sites by more than 100 K or several tens of kJ per mol being thermally stable up to more than 500 K. Surprisingly, the multidentate adsorption is remarkably sensitive to the adsorption conditions and especially does not occur for elevated adsorption temperatures. Curiously enough, low adsorption temperatures thus yield high-temperature desorption species. Our findings are particularly important for the understanding of (photo-)chemical reactions, which usually requires an extensive knowledge of adsorbate-substrate interactions and the bonding situation toward the catalysts surface.
9. Jessica Kräuter, Lars Mohrhusen, Fabian Waidhas, Olaf Brummel, Jörg Libuda, Katharina Al-Shamery: Photoconversion of 2-Propanol on Rutile Titania: A Combined Liquid-Phase and Surface Science Study
J. Phys. Chem. C 125, 6, 3355-3367 (2021).

A fundamental reaction in industries for producing aldehydes and ketones is the partial oxidation of alcohols. As a model reaction, we investigated the photo-oxidation of 2-propanol on rutile titania, which is a promising chemically nontoxic photocatalyst. Photochemical infrared reflection absorption spectroscopy (PC-IRRAS) was used to study the reaction on powder catalysts in the liquid phase (neat liquid and dissolved in dichloromethane). We compare these results with polarized Fourier transform (FT)-IRRAS and temperature-programmed desorption (TPD) experiments on rutile TiO2(110) single crystals in ultrahigh vacuum (UHV). Our in situ liquid-phase experiments showed that 2-propanol converts into acetone on rutile powders, which is in accordance with previous ex situ studies. Mass transport limitations are the key to avoid total oxidation. However, the yield of acetone is limited. We identified water formed as a byproduct and suspected that water might block the active sites. To elucidate possible reaction mechanisms, further experiments were performed on rutile TiO2(110) single crystals in the presence and absence of oxygen and UV irradiation under UHV conditions. Here, we obtained further insights into the elementary steps of the different 2-propanol reactions. We demonstrated that acetone desorbs from a diolate species, which forms in the presence of oxygen under UV irradiation at temperatures around 200 K. Furthermore, propane was identified for the first time as a new thermally activated deoxygenation product besides the simultaneously formed, formally reported, propene. Propene formation is quenched by UV irradiation. Active site blocking by water is confirmed by TPD and polarized FT-IRRAS measurements.
8. Lars Mohrhusen*, Maximilian Grebien, Katharina Al-Shamery: Electron Transfer in Oxide-Oxide Cocatalysts: Interaction of Tungsten Oxide Clusters with Ti3+ States in Rutile TiO2
J. Phys. Chem. C 124, 43, 23661-23673 (2020).
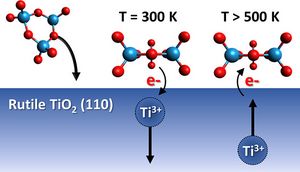
Heterogeneous (photo)catalysts are often complex mixtures of different nanostructured oxidic compounds. Chemical and electronic interactions within such combined materials may play a key role in improving the performance in technological applications but are difficult to investigate under technical conditions. This work presents a systematic study of the interactions between tungsten oxide clusters and the underlying rutile TiO2 (110) surface in special consideration of point defects such as Ti3+ interstitials. Using electron beam evaporation from WO3 powder, stoichiometric (WO3)n, and oxygen-deficient (WO3-x)n, tungsten oxide clusters are produced simultaneously. Based on cluster coverage- and temperature-dependent X-ray photoelectron spectroscopy studies, the formation of a surface layer of stoichiometric and oxygen-deficient tungsten oxide clusters is shown, and the formation of mixed oxides can be excluded. For the first layer up to 7.1 WO3 nm-2, stoichiometric clusters are dominant at the TiO2 surface. The lack of W5+ indicates an electron transfer from the clusters toward the substrate under formation of Ti3+ interstitials. Furthermore, we found at elevated temperatures relevant for catalytic reactions that the tungsten oxide clusters are more stable on TiO2 surfaces than on other substrates such as the silicon oxide layer of Si wafers. Up to 900 K, only slight changes were observed on titania. We observed an accumulation of Ti3+ at the TiO2 surface between 500 and 800 K in the case of high bulk Ti3+ content in TiO2. As the Ti3+ accumulation is accompanied by significant changes of the W 4f signals, we suggest an interaction between these sites under a possible generation of surface fields or an anionic [(WO3)n]z--like cluster by electron transfer from Ti3+.
7. Lars Mohrhusen*, Jessica Kräuter, Michael Willsm, and Katharina Al-Shamery: Argon Embedded by Ion Bombardment: Relevance of Hidden Dopants in Rutile TiO2
J. Phys. Chem. C 123, 33, 20434-20442 (2019).

To obtain a mechanistic understanding of occurring processes on oxide surfaces at the atomic level, systematic studies under ultra-high vacuum (UHV) conditions on single crystalline surfaces are commonly used. Usually, the sample preparation protocol for these surfaces includes argon-ion bombardment followed by annealing at elevated temperatures. For reducible metal oxides, this leads to a significant reduction of the surface. Up to now, the particular role of the remaining argon in the subsequent formation of clean surfaces and the possible incorporation of argon into the crystal lattice as a dopant are typically neglected or remain unclear. This work presents combined, temperature-dependent X-ray photoelectron spectroscopy and a low-energy electron diffraction study under UHV conditions of the bulk-assisted reoxidation and restructuring of the rutile TiO2(110) single crystal surface after argon-ion bombardment. The formation of an ordered and reoxidized (110)(1 × 1) surface is accompanied by a stepwise desorption of argon from the sample. Furthermore, we present a systematic study of the incorporation of argon in the rutile crystal as well as the diffusion and desorption of argon from these samples. By following the temperature-dependent Ar 2p photoelectron spectra, the change of the electronic environment of embedded argon is elucidated, demonstrating the interaction with reduced Ti cations. Hence, residual argon (in case of Ar+) possibly acts as a strong oxidant or induces significant lattice distortions. Our results show that residual argon from the sample preparation is an important hidden dopant and needs to be considered in the evaluation of typical studies on oxide surfaces under UHV conditions in future work.
6. Jessica Kräuter, Lars Mohrhusen, Tim Thiedemann, Michael Willms and Katharina Al-Shamery:Activation of Small Organic Molecules on Ti2+-Rich TiO2 Surfaces: Deoxygenation vs. C-C Coupling
Z. Naturforsch. 74(8)a: 697-707 (2019).
5. Jingwei Chen, Lars Mohrhusen, Ghulam Ali, Shaohui Li, Kyung Yoon Chung, Katharina Al-Shamery, Pooi See Lee: Electrochemical Mechanism Investigation of Cu2MoS4 Hollow Nanospheres for Fast and Stable Sodium Ion Storage
Adv. funct. Mater. 29, 1807753 (2019).
Sodium ion batteries (SIBs) are promising alternatives to lithium ion batteries with advantages of cost effectiveness. Metal sulfides as emerging SIB anodes have relatively high electronic conductivity and high theoretical capacity, however, large volume change during electrochemical testing often leads to unsatisfactory electrochemical performance. Herein bimetallic sulfides Cu2MoS4 (CMS) with layered crystal structures are prepared with glucose addition (CMS1), resulting in the formation of hollow nanospheres that endow large interlayer spacing, benefitting the rate performance and cycling stability. The electrochemical mechanisms of CMS1 are investigated using ex situ X-ray photoelectron spectroscopy and in situ X-ray absorption spectroscopy, revealing the conversion-based mechanism in carbonate electrolyte and intercalation-based mechanism in ether-electrolyte, thus allowing fast and reversible Na+ storage. With further introduction of reduced graphene oxide (rGO), CMS1-rGO composites are obtained, maintaining the hollow structure of CMS1. CMS1-rGO delivers excellent rate performance (258 mAh g-1 at 50 mA g-1 and 131.9 mAh g-1 at 5000 mA g-1) and notably enhanced cycling stability (95.6% after 2000 cycles). A full cell SIB is assembled by coupling CMS1-rGO with Na3V2(PO4)3-based cathode, delivering excellent cycling stability (75.5% after 500 cycles). The excellent rate performance and cycling stability emphasize the advantage of CMS1-rGO toward advanced SIB full cells assembly.
4. Xuefei Gong, Jingwei Chen, Shaohui Li, Lars Mohrhusen, Katharina Al-Shamery, Pooi See Lee: Vanadium Oxide Nanosheets for Flexible Dendrite-Free Hybrid Aluminum-Lithium-Ion Batteries with Excellent Cycling Performance
Batteries & Supercaps 2, 205-212 (2018).
Aluminum (Al)-ion batteries can be an attractive alternative to lithium-ion batteries because of low costs, high volumetric capacities and dendrite-free formation when Al is used as anode. However, there are limited cathode materials for Al-ion batteries that can deliver satisfactory electrochemical performance, especially cycling stability. The major reason for that is the sluggish kinetics of ion intercalation/deintercalation, resulting from large coulombic attraction between Al3+ and cathodes. Herein, a concept of hybrid Al-Li-ion batteries is proposed to circumvent the poor Al3+ ions insertion/extraction kinetics in Al-ion batteries, and maintain the dendrite-free characteristics of Al-ion batteries. The high volumetric capacity (32.5 mAh/cm3 at 100 mA/cm3, based on the total volume of cathode), enhanced rate capability (21.5 mAh/cm3 at 1000 mA/cm3) and excellent cycling performance (70.1 % retention after 3000 cycles) have been achieved in the hybrid Al-Li-ion battery composed of vanadium oxide nanosheets on carbon fibers as cathode and Al as anode in a mixed [EMIM][Cl]/AlCl3/LiCl electrolyte. Combining with the good flexibility of cathode and anode, the hybrid Al-Li-ion battery maintains structural and capacity stability under different bending angles. This study unveils a safe, cost-effective and flexible hybrid Al-Li-ion battery that presents highly competitive advantages among various energy storage devices.
3. Milena Osmic, Lars Mohrhusen and Katharina Al-Shamery: Bulk Defect Dependence of Low-Temperature Partial Oxidation of Methanol and High-Temperature Hydrocarbon Formation on Rutile TiO2 (110)
J. Phys. Chem. C 123, 13, 7615-7626 (2019).
Titanium dioxide (TiO2) is among the most studied model (photo)catalyst materials. The influence of surface point defects, like oxygen vacancies and particularly bulk defects such as Ti3+ interstitials, is usually underestimated or even ignored. We present a systematic study under well-defined UHV conditions illustrating the importance of such defects for the thermal reaction of methanol at the rutile TiO2 (110) single crystal surface by using temperature-programmed reaction spectroscopy (TPRS) and Fourier-transform infrared reflection-absorption spectroscopy (FT-IRRAS). It will be shown that the population of different reaction pathways, namely, the partial oxidation of methanol to formaldehyde and the deoxygenation forming hydrocarbons, especially methane, depends on the bulk defect density and the presence or absence of oxygen adsorbates. While at elevated temperatures molecular desorption is pronounced for the less defective substrates, for higher reduction grades the high-temperature deoxygenation channel via methoxy intermediates is favored. In addition, preadsorption of oxygen enables low- and high-temperature partial oxidation forming formaldehyde, likely from a dioxomethylene-like adsorbate.
2. Michael Siemer, Lars Mohrhusen, Maximilian Grebien and Katharina Al-Shamery: Amine Capped Gold Colloids at Oxidic Supports: Their Electronic Interactions
Z. Phys. Chem. 233, 69-84 (2019).
Colloidal deposition of noble metal nanoparticles on oxidic supports is a recent approach for the fabrication of heterogeneous catalyst materials. We present studies on the interaction of different amine ligands with gold nanoparticles before and after deposition on several oxidic supports (titania, silica, alumina, magnesia or zinc oxide), using X-ray photoelectron and Auger spectroscopy, and high-resolution transmission electron microscopy. The adsorption of amines on thin gold films as well as on nanoparticles leads to a decrease in metal photoelectron binding energies. Usually, this is explained by donor-acceptor interactions via the amine group. By additional analysis of Auger signals, which are more sensitive to changes in the oxidation state than photoelectron spectra, we demonstrate that these shifts are due to a final state effect, namely, the increased photoelectron hole screening in presence of amine adsorbates. It will be shown that this effect is not sensitive neither to the nanoparticle size nor the sterical properties of the capping amine. After deposition on oxide supports, the photoelectron binding energies are even further decreased. The presented findings exhibit that care has to be taken to interpret binding energy shifts simply with charging, which has impact on understanding the local electronic situation on the surface of metal-loaded oxides, crucial for heterogeneous catalysis.
1. Lars Mohrhusen* and Milena Osmic: Sterical ligand stabilization of nanocrystals versus electrostatic shielding by ionic compounds: a principle model study with TEM and XPS
RSC Adv. 7, 12897-12907 (2017).
Colloidal metal nanoparticles are usually fabricated via the reduction of metal salt precursor compounds in liquid phase. To prevent agglomeration, organic capping agents are used. Commonly, it is neglected that ionic compounds may coadsorb when present in the mixture. However, we shall show that ionic adsorbates play a key role in size and growth control. We present a universal case study on gold nanoparticles with various amine ligands to reveal two competitive stabilization mechanisms via quantitative X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM). Gold nanocrystals were obtained starting with a single phase room temperature synthesis. The stabilization turned out to be a combination of sterical and electrostatic shielding depending on the ligand molecule properties. We can adjust the ratio of both contributions via simple liquid phase ligand exchange procedures at moderate temperatures as shown with XPS. Apparently, ions further have the power to steer different ripening processes. HR-TEM studies proved that there is no influence on nanoparticle morphology during heat treatment or ligand exchange. Introducing a lack of stabilization by weaker sterical ligands offers an auspicious new way for the synthesis of porous nanomaterials. The novel findings illustrate that electrostatic stabilization by coadsorbed ionic compounds can play a crucial role in understanding various experimental results and thus the colloidal synthesis of nanomaterials in general appears in a new light.

