Contact
Mailing Address
Visitors
Langmuir-Blodgett Transfer Technique
Instrumentation

Langmuir-Blodget trough with film balance and motorized barriers (KSV).
Monolayers at the Water-Air Interface
Irving Langmuir demonstrated that amphiphilic molecules have the ability to form stable monomolecular layers at the water-air interface. An amphiphilic molecule has a specific structure. It is composed of two parts: non-polar "tail", which usually is (are) hydrocarbon chain(s) and the polar group "head group". Head groups are various polar groups, e.g., hydroxyl, amine, carboxylic, lipids etc.
The amphiphilic compound is dissolved in an easily evaporating solvent (e.g., chloroform) and placed at the water-air interface (subphase) in the Langmuir trough (Figure 1). After solvent evaporation, movable barriers start to approach each other and compress the monolayer at the interface.
At the beginning of compression, the amphiphilic molecules are separated and they do not interact between each other (gas-like state). During compression, the barriers reduce the area available per molecule causing stronger intermolecular interactions. Formation of the monolayer is followed by the measurement of the surface tension that increases upon compression. Surface pressure (π) is the difference between the surface tension of clean subphase (γ0) and of the surface tension at the air-water interface covered by the monoalyer (γ):
![]()
An experimental result showing changes of the surface pressure as the function of the molecular area is called an isotherm. An example is shown on Figure 2.
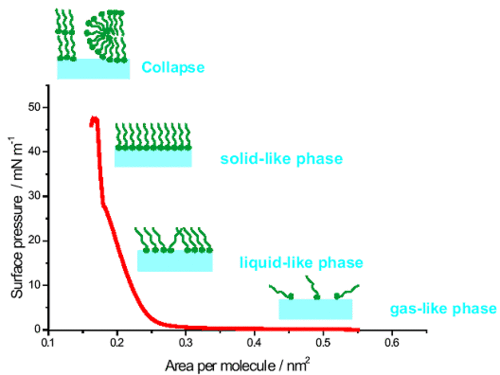
The isotherm shows some inflection points which are related to phase transitions in the monolayer. At the beginning of monolayer compression intermolecular distances are large and the interactions are negligible. The surface pressure is close to zero and the monolayer exists in two-dimensional gas-like phase. The decrease of the area per molecule causes an increase of interactions and the transitions to a liquid-like state. In some cases a liquid expanded (LE) and liquid condensed (LC) can be distinguished. Finally, solid-like phase is formed, inwhich the molecules are densely packed as in a crystal. Further compression of the monolayer causes its destruction, the collapse.
The compressibility modulus (Ks) can be calculated from the slope of the isotherm. |
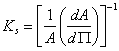
where A is the area per molecule and π is the surface pressure.
Higher values of the compressibility modulus indicate low interfacial elasticity of the monolayer and in general they occur at high surface pressures. The compressibility modulus values are useful for characterization of the physical state of the monolayer. In the LE state Ks values vary from 12.5 to 50 mN m-1, in the LC from 100 to 250 mN m-1. Transition to the solid-like phase (S) causes an increase of the Ks up to 2000 mN m-1.
Phase transitions and aggregate formation in the monolayer are caused by intermolecular interactions. In order to reduce these interactions, multicomponent monolayers are investigated. Monolayers composed of large amphiphilic molecules [e.g., macorcycles (porphirines, azocronehters), Q10 coenzyme or polymers] often form aggregates. In multicompoent films, small amphiphilic molecules (acids, alcohols, amines or lipids) are used as "solvents", which reduce intermolecular interactions.
In a mixed monolayer, changes of the surface pressure and of the area per molecules are calculated from additivity rule. They are compared with experimentally obtained values and their difference is called excess pressure (πex) and excess area (Aex), respectively:
![]()
![]()
Aex is a measure of miscibility and interactions between components of a mixed monolayer. The origin of interactions in mixed monolayers is rooted inthe chemical structure of film components, having a hydrophobic "tail" and a hydrophilic "head-group". Two different effects influence the strength of intermolecular interactions in the monolayer. One of them is due to attractive forces between hydrocarbon chains resulting in the formation of aggregates such as micelles and multilayers. The other effect is due to interacting polar groups. In the a mixed monolayer, "head-grouos" may be of the same or of different kind, e.g., non-ionic, cationic or anionic. The electric charge of the polar group causes them to attract or repulse. These interactions are much stronger in comparison to hydrocarbon chains association and, therefore, are mostly responsible for deviations from ideality. Figure 3 shows possible changes of the measured and ideal area in the function of the mole fraction of one of the monolayer component. When the experimental points follow the calculated line, no intermolecular interactions are detected between compounds in the monolayer (Figure 3A). Positive values of the excess area indicate repulsion between monolayer components (Figure 3B). Negative values of the excess area indicate attractions, condensation, including accommodation and hydration changes (Figure 3C).
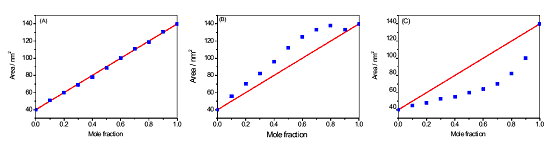
Transfer on Solid Substrates
The monolayer formed at the water-air interface can be transferred onto an solid substrate by the Langmuir-Blodgett (LB) or the Langmuir-Schaeffer (LS) methods.
Before transfer, a monolayer is formed at the water-air interface and it is compressed to a choosed surface pressure, at which the transfer will take place. The substrate used for transfer can be hydrophilic (e.g., glass, mica, or metal oxides surfaces) or hydrophobic (mercury, glassy carbon).
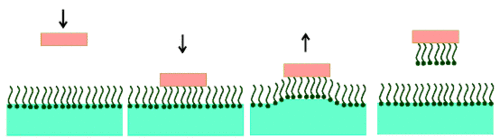
In the Langmuir-Blodgett technique, the substrate is vertically immersed (Figure 4A) or withdrawn (Figure 4B) through the interface covered by the monolayer.
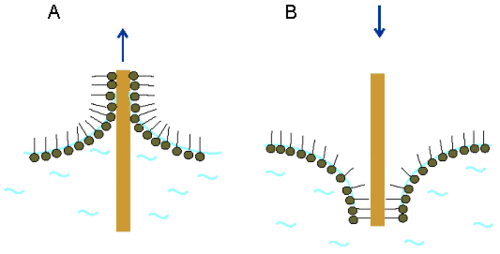
Depending on the nature of the substrate, the used molecules orient differently on the solid surface. When a hydrophilic substrate is used, it is withdrawn from the aqueous subphase through the interface covered by the monolayer. In this case the hydrophilic polar head group orients towards the substrate. As a result, the surface of the substrate is covered by the monolayer becomes hydrophobic (Figure 4A). A hydrophobic substrate is immersed from the air through the interface into the subphase. Here, the hydrophobic chains are oriented towards the substrate. After the transfer, the surface becomes hydrophilic in nature (Figure 4B). |
The transfer ratio (TR) is the indicator of the successful transfer of the monolayer from aqueous subphase on the solid substrate. The TR is the ratio of the area (AL) at the water-air interface, which is reduced during transfer at constant surface pressure to the area of the substrate (AS) which is covered by the monolayer.
![]()
For a quantitative transfer of the monolayer the transfer ratio should be equal to unity.
In order to obtain TR = 1, various surface pressures and various speeds of the transfer should be used. In general the higher surface pressure of the transfer the higher speed of the transfer. Usually withdrawing speed is higher than immersing. The LB transfer can also be realized under an angle which is different than 90 degree to the interface. Some molecules, e.g., cyclames and α,ω-hydroxyalkanethioles, can be quantitiatievly transferred onto the solid substrate only when the substrate is tilted by 45° or 60°. Sometimes the transfer can be realized only when the substrate horizontally touches the interface covered by the monolayer. It is Langmuir-Schaeffer technique, also called horizontal touch technique. Figure 5 presents its principles.
The substrate slowly approaches the interface with the previously prepared monolayer. Next, it is brought in contact with the organic film and slowly lifted-up from the interface. In these monolayers the hydrophobic part of the amphiphilic molecule is turned towards the substrate.
Formation of Multilayers
The transfer of the first monolayer onto the solid substrate is dependent on the interactions between the substrate and the amphiphilic molecule. The transfer of successive layers does not depend on substrate - molecule interactions but on interactions between hydrophobic and hydrophilic parts of the amphiphile. Figure 6 shows different arrangement in LB multilayers.
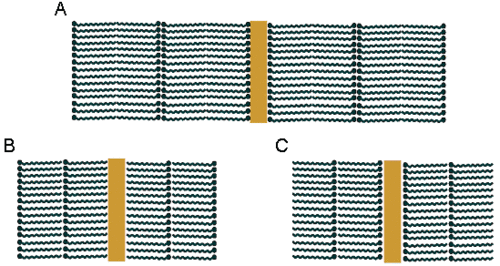
The Y type multilayers are formed when successive withdrawing and immersing result in TR ≈ 1. In these films, molecules have the same arrangement like in the natural biological membranes.
The X type multilayers are formed when TR ≈ 1 only for immersing while the TR is zerro for withdrawing. These layers form compounds, in which the hydrophilic part of the molecules does not have a strong polar character, e.g., esters. Usually, the contact angle is higher than 90° and as a result only immersing yields successful transfer.
In Z trype multilayers, molecules have an opposite orientation than in X type monolayers. TR is unity only for withdrawing of the substrate. This kind of multilayers is typically obtained for amphiphilic molecules possessing large polar groups that are often charged. Here, the contact angle is lower than 90°, thus only substrate withdrawing results in quantitative transfer of the monolayer.

