Project-ID
Lead: Maren Striebel (ICBM), Uwe John (AWI), Antonia Ahme (AWI), Anika Happe (ICBM)
Involved from PEL: Maren Striebel, Anika Happe
Collaborators: Antonia Ahme (AWI), Uwe John (AWI), Marco Jabalera Cabrerizo (University of Vigo, AQUACOSM-Plus), Markus Olsson (Stockholm University, AQUACOSM-Plus), Simon Hasselø-Kline (University of Oslo, AQUACOSM-Plus), Alexander Sentimenti (TU Munich, AWI), Ruben Schulte-Hillen (TU Munich, AWI), Nancy Kühne (AWI), Jakob Giesler (AWI).
Funded by: The project is funded by AQUACOSM-plus (Project No. 871081) through the European Commission EU H2020-INFRAIA and by the Helmholtz Research Programme "Changing Earth, Sustaining our Future" (Theme 6 "Marine and Polar Life" in Subtheme 6.2 "Adaptation of marine life: from genes to ecosystems") of the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Germany.
Duration: March-April 2022
Current Projects 08
AQUACOSM-Plus: SupOpTrons - Unravelling the effects of supra-optimal temperatures and varying N:P ratios on North Sea microplankton communities
Microplankton communities play a fundamental role in the North Sea ecosystem. Especially during the spring bloom, they form a starting point of marine energy flux by providing organic matter for higher trophic levels, produce oxygen for heterotrophic processes and contribute to the biogeochemical cycling of elements such as carbon, nitrogen and phosphorus. Currently, they are facing drastic alterations in terms of their abiotic environment as ocean temperatures are increasing and N:P ratios are changing (IPCC, 2021). Both factors affect the various groups within unicellular planktonic communities differently and thus shape the outcome of competition, facilitation, herbivory and parasitism (Boyd et al., 2018). In light of climate change, it is particularly interesting to look at tipping points, e.g., how communities will react when they are exposed to supra-optimal temperatures. The project aims to capture changes on several levels of the complex architecture and high intra- and interspecific diversity of natural microplankton communities exposed to supra-optimal temperatures and varying N:P ratios. For this, sea water from Helgoland Roads has been incubated in the Planktotron mesocosm facility at three temperatures with four replicates each which runs simultaneously with bottle-incubations with varying N:P ratios in controlled temperature rooms. In addition to standard parameters such as pH, alkalinity, chlorophyll and nutrients, we are interested in the composition of the protist community, the importance of intraspecific diversity, the impact of micro-grazing, the microbiome, the metatranscriptome as a link to functional biodiversity and process understanding as well as changes in dissolved and particulate organic matter.
BOYD, P. W., COLLINS, S., DUPONT, S., et al. 2018. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—A review. Global Change Biology,24,2239-2261.
IPCC 2021. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)].
The Team

(from left: Nancy Kühne, Ruben Schulte-Hillen, Alexander Sentimenti, Marco J. Cabrerizo, Antonia Ahme, Simon Hasselø-Kline, Anika Happe, Jakob Giesler, Markus Olsson)
Overview of the different stations
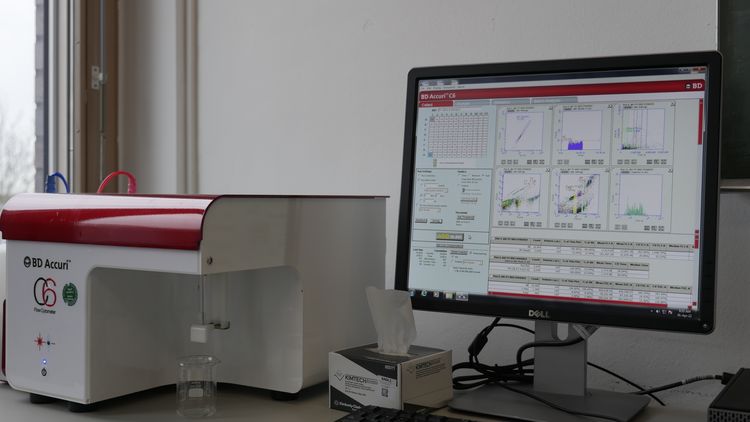
| Station 1: Flow cytometry (Antonia Ahme)
|
| Station 2: Single-cell isolation (Antonia Ahme)
|
| Station 3: Fume hood (Antonia Ahme, Ruben Schulte-Hillen)
|
| Station 4: N:P bottle incubations (Anika Happe) Simultaneously to filling up the Planktotrons, the same seawater
|
| Station 5: DNA Filtration (Alexander Sentimenti)
|
| Station 6: Micrograzing (Marco J. Cabrerizo)
|
| Station 7: Chlorophyll filtration (Markus Olsson)
|
 | Station 8: Daily small sampling (Nancy Kühne)
|
| Station 9: Thermal Performance Curves (Simon Hasselø-Kline)
|
 | Station 10: Nutrient Limitation Assays (Simon Hasselø-Kline) Life in the ocean is dependent on a variety of different nutrients and elements. Without the cocktail of many nutrients, planktonic life would be unable to grow. Human influence affects the balance of nutrients in the ocean, which can determine which planktonic organisms can grow. We investigate how nutrients may limit or enhance growth, by adding nitrogen, phosphorous and/or silicate to the water samples. The growth of the organisms in the nutrient added water is then measured over time using a plate reader, which allows us to estimate how the concentration of organisms changes over time.
|



![[Translate to English:]](/f/5/_processed_/3/2/csm_ICBM-Logo-transparent-_91fe1c6774.png)