Electrochemistry and Catalysis at Liquid-Liquid Interfaces
Contact
Mailing Address
Visitors
Electrochemistry and Catalysis at Liquid-Liquid Interfaces
by Shokoufeh Rastgar
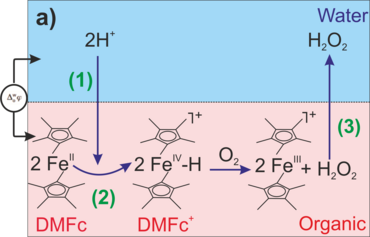
Liquid/liquid (L/L) interfaces formed between two immiscible electrolyte solutions (ITIES) not only enable the separation of compounds by their differential solubility but also to control and to monitor the transfer processes of ions, electrons and neutral species across such interfaces at the same time. Such systems can thus be considered as a biomimetic but simplified platform to study vital reactions in compartmentalized media.1 This includes reaction of partners in immiscible solvents at the interface to synthesize organic substrates, and energy-related proton-coupled electron transfer reactions such as oxygen reduction reaction (ORR) to produce hydrogen peroxide (Figure 1a)2 and water splitting reactions3 by focusing on producing either dioxygen (O2)4 or hydrogen (H2).5,6
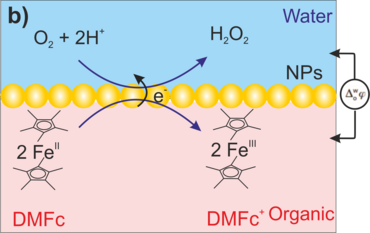
Figure 1. Reaction schemes of ORR at a L/L interface in the presence of organic e--donor, Decamethylferrocen; (a) potential-driven H+ transfer (1) followed by homogenous reaction to produce H2O2 (2) and its extraction into aqueous phase (3); (b) interfacial reaction of H+ and O2 to produce H2O2 with a nanocatalyst.
The L/L interfaces are soft, defect-free and self-healing. The catalyst units, i.e., metal or metal oxide nanostructures can be practically assembled at such interfaces (for example, Figure 1b), and served as bipolar electrodes to facilitate catalysis via direct interfacial electron transfer, between an organic-soluble electron donor species, e.g., metallocenes and molecular O2 driven by the potential drop (∆aq/of) at the soft “electrode” surface being adjustable either electrochemically or chemically. Of particular interest is that the nanocatalyst can easily be exchanged at L/L interface. In conztrast, no solid electrode can ever provide this degree of flexibility and deactivation of such units has remained a major problem in almost all electrochemical oxygen reactions.
Our contribution to the field
Although catalysts are largely used at L/L processes,7,8 detailed studies in order to separate catalytic reactivity and the product selectivity have remain restricted over the decades. This stems from technically inaccessibility of the combined transfer processes of ions, electrons and neutral products accompanying the photocatalytic reactions at the L/L interface. We have developed different configurations of scanning electrochemical microscopy (SECM) with integrated L/L interfaces to explore those entangled processes independently.
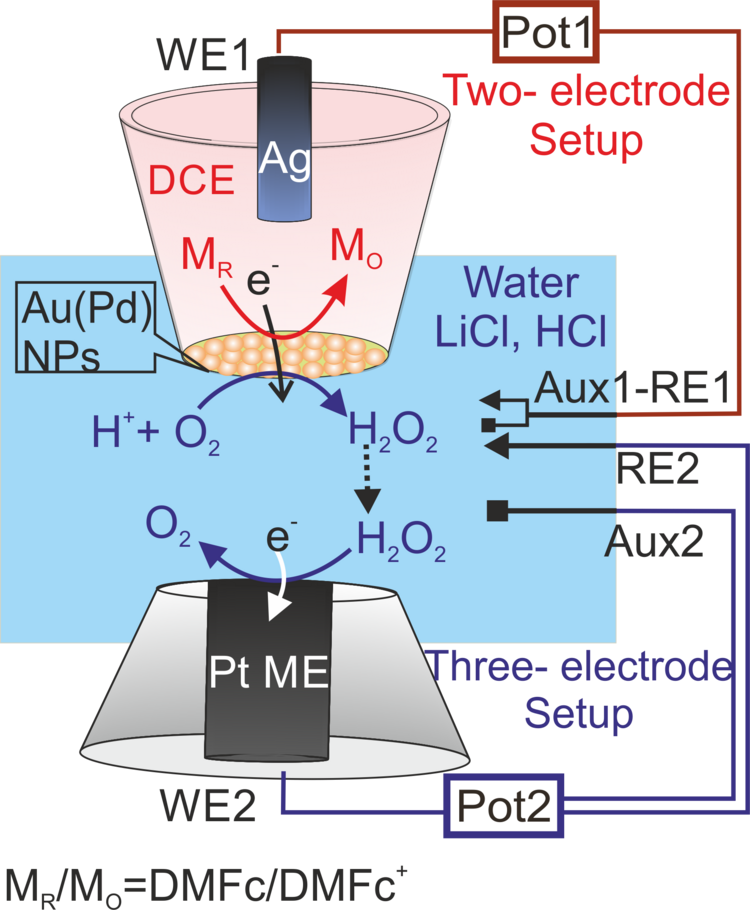
To follow the mentioned goals, SECM experiments have been designed in the substrate-generation/tip-collection (SG/TC) mode. 9,10 A key aspect is the mechanical stabilization of the L/L interface at the orifice of a micropipette facilitating its positioning in very close and defined distance to a microelectrode for product detection. It was applied for the oxygen reduction reaction at L/L interface systems with the metal alkaline cations as the phase-transfer catalysts demonstrating that H+ transfer exclusively plays a key role in synthesis protocol of the hydrogen peroxide product (as shown in Figure 1a).9 In this context, the project is currently running to determine oxygen reduction reaction reactivity at gold or palladium metallic (or bimetallic) nanoparticles assembled at the L/L interface, simultaneous to H2O2 detection (Figure 2).
Moreover, we extended the application of the proposed approach by coupling light pulses to such a configuration to locally follow the photochemical catalytic reactions at BiVO4 nanoparticles-modified L/L interface10-12 and simultaneous detection of the products of water oxidation reaction, either O2 (as a main product, Figure 3a,3b)11 or the reactive oxygen species (e.g., adsorbed OH·) (Figure 3c, 3d). 10 For the latter case, we used the surface interrogation mode (SI) of SECM at a L/L interface for studying the photogenerated surface intermediates during water oxidation at BiVO4 nanoparticles.10 (Figure 3c). The SECMx software was expanded in our group to apply predefined potential and light pulses, switch the connection to the electrode and record current transients during light and potential pulses (Figure 3d). In those cases, the potential drop at L/L interface was chemically tuned.
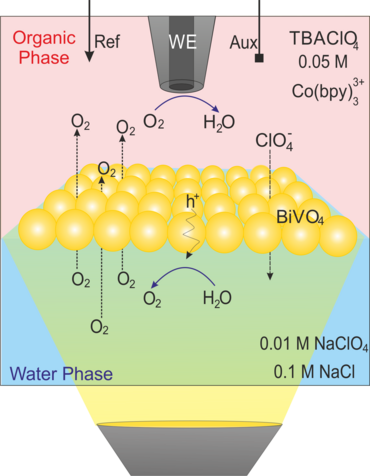
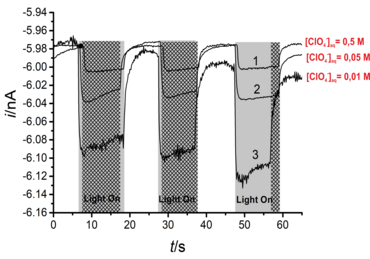
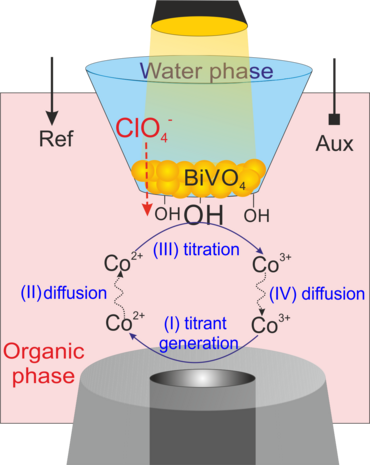
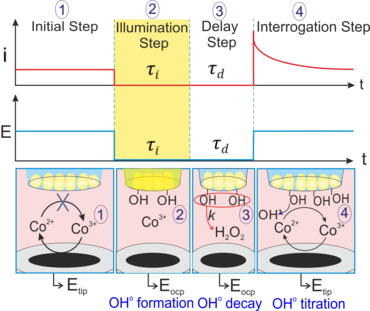
Figure 3. (a) Schematic representation of SECM SG/TC mode for local detection of photocatalytically generated O2; (b) Transient currents for reduction of photogenerated O2 by ClO4− ion transfer at different concentration of ClO4− in aqueous phase. Further details in our published work;11 (c) schematic representation of SI-SECM setup for the quantitative assessment of surface intermediates OH•ads of photochemical reaction and their decay process. [Co(bpy)3]3+ was used as a titrant; (d) experimental sequence for the potential and light pulses applying and the current transients recording for the generation and detection of OH•ads on the BiVO4 surface.
References
- K. Piradashvili, E. M. Alexandrino, F. R. Wurm und K. Landfester, Reactions and Polymerizations at the Liquid-Liquid Interface, Chem. Rev., 2016, 116, 2141-2169.
- B. Su, R. P. Nia, F. Li, M. Hojeij, M. Prudent, C. Corminboeuf, Z. Samec und H. H. Girault, H O22 Generation by Decamethylferrocene at a Liquid|Liquid Interface, Angew. Chem. Int. Ed., 2008, 47, 4675-4678.
- M. D. Scanlon, P. Peljo, L. Rivier, H. Vrubel und H. H. Girault, Mediated Water Electrolysis in Biphasic Systems, Phys. Chem. Chem. Phys., 2017, 19, 22700-22710.
- S. N. M. Betancourt, J. S. Riva, J. G. Uranga, A. J. Olaya und H. H. Girault, Visible-light Driven Water Oxidation and Oxygen Production at Soft Interfaces Chem. Commun. 2022, 58, 3965-3968.
- L. Rivier, P. Peljo, L. A. C. Vannay, G. C. Gschwend, M. A. Méndez, C. Corminboeuf, M. D. Scanlon und H. H. Girault, Photoproduction of Hydrogen by Decamethylruthenocene Combined with Electrochemical Recycling, Angew. Chem. Int. Ed., 2017, 56, 2324-2327.
- P. Ge, M. Hojeij, M. D. Scanlon und H.H. Girault, Photo-recycling the Sacrificial Electron Donor: Towards Sustainable Hydrogen Evolution in a Biphasic System, ChemPhysChem, 2020, 21, 2630-2633.
- A. G. Quijano, G. Herzog, P. Peljo, M.D. Scanlon, Electrocatalysis at the Polarised Interface Between Two Immiscible Electrolyte Solutions, Curr. Opin. Electrochem., 2023, 38,101212, DOI:10.1016/j.coelec.2023.101212.
- M. Opallo, K. Dusilo, M. Warczak, J.Kalisz, Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction at Polarizable Liquid|Liquid Interfaces, ChemElectroChem, 2022, 9, e202200513.
Own Contributions to the Research Field
- S. Rastgar, K. Teixeira Santos, C. A. Angelucci, G. Wittstock
Catalytic activity of alkali metal cations for chemical oxygen reduction reaction in a biphasic liquid system probed by scanning electrochemical microscopy
Chem. Eur. J. 2020, 26, 10882-10890. Abstract & Link (Open Access)
- S. Rastgar, G. Wittstock
In Situ Microtitration of Intermediates of Water Oxidation Reaction at Nanoparticles Assembled at Water/Oil Interfaces
J. Phys. Chem. C, 2018, 122, 12963−12969. Abstract & Link
- S. Rastgar, G. Wittstock
Characterization of Photoactivity of Nanostructured BiVO4 at Polarized Liquid-Liquid Interfaces by Scanning Electrochemical Microscopy
J. Phys. Chem. C 2017, 121, 25961-25948. Abstract & Link
- S. Rastgar, M. Pilarski, G. Wittstock
Polarized liquid-liquid interface meets visible light-driven catalytic water oxidation
Chem. Commun. 2016, 52, 11382-11385. Abstract & Link - W. Adamiak, J. Jedraszko, W. Nogala, M. Jönsson-Niedziolka, S. Dongmo, G. Wittstock, H.H. Girault, M.Opallo
A Simple Liquid–Liquid Biphasic System for Hydrogen Peroxide Generation
J. Phys. Chem. C, 2015, 119 (34), 20011–20015, Abstract & Link - J. Jedraszko, W. Nogala, W. Adamiak, S. Dongmo, G. Wittstock, H. H. Girault, M. Opallo
Catalysis at room temperature ionic liquid|water interface: H2O2 generation
Chem. Commun. 2015, 51, 6851-6853, Abstract & Link