Contact
Mailing Address
Visitors
Electrocatalysis and Transport in Nanoporous Gold
von Mareike Hänsch und Gunther Wittstock
Nanoporous bulk materials have a wide range of applications due to their combination of a very high surface area and an interconnected pore system, which is well accessible for reactive species. Nanoporous gold has the particlar advantage of chemical stability, specific (electro)catalytic properties and tunable structure and surface composition. Therefore, nanoporous gold play an important role when the real surface area of the material matters. This applies for catalysis, actuation, energy storage and energy conversion.
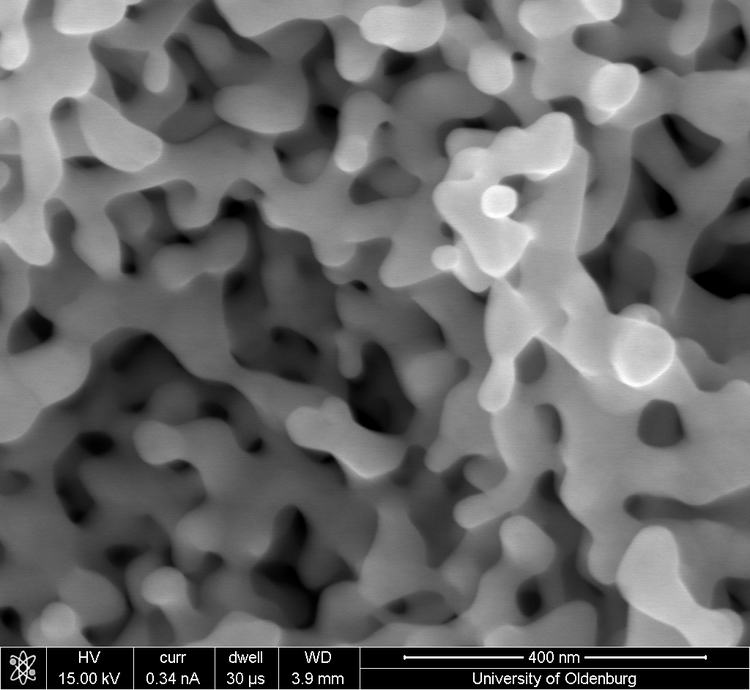
Nanoporous gold exhibits a bicontinuous structure of nanometer sized ligaments and pores. In contrast to well-defined (e.g., flat single crystals) electrodes which are already well described the understanding of nanoporous electrodes lacks considerably behind. This work, being part of the DFG Research Unit FOR 2213, focuses on transport processes in- and outside the nanoporous network and on the electrocatalysis of methanol oxidation as a model reaction. The aim is to obtain a fundamental understanding of the complex interplay of structure and properties and to use this knowledge to tailor new catalysts on a rational basis. The nanoporous material will be obtained by dealloying a less noble metal (e.g. Ag) from a Ag-Au-alloy. It will be possible to tailor the morphology of the material by using different dealloying processes resulting in distinct ligament and pore sizes. This and starting materials with varying ratios of the two metals can be used to obtain different residual contents of the less noble metal which can have a major influence on electrocatalytic properties and the surface reactivity in general. Another way to tailor the surface composition of the material is through UPD (underpotential deposition) of foreign metals. The final surface composition after a possible segregation of residual atoms or alloy formation of foreign metals can be investigated with XPS (X-ray photoelectron spectroscopy). The influence on the transport processes inside the nanoporous gold will be investigated with SECM (scanning electrochemical microscopy) and CLSM (confocal laser scanning microscopy). Since the electrocatalytic reaction of methanol oxidation does not need molecular oxygen as oxidizer, it ise of particular interest to compare it with the methanol oxidation in liquid and gas phases (studied in other sub-projects) with respect to the the influence of transport behavior and surface reactivity.
Own Contribution to the Field
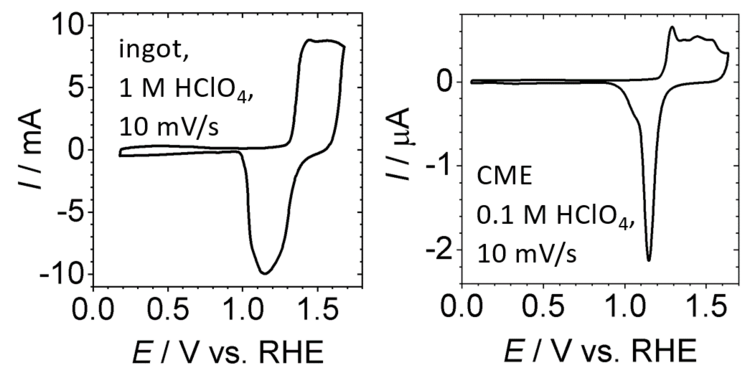
Our group used cavity microelectrodes to study the voltammetric behavior of nanoporous gold. A cavity microelectrode is obtained starting from a conventional electrode, which is etched to obtain a recessed electrode. Pulverized nanoporous gold is filled into the cavity. This is possible without destroying the nanostructure of the material. Due to the oevall reduced currents, the voltammetric signals are very well resolved and allowed details to observe in underpotential deposition adn electrocatalysis that were not accessible before. Site directed underpotential deposition could be used to block specific surface sites for electrocatalysis or to introduce new elements on the surface of the ligaments to observe their impact on electrocatalysis.
Reviews
- G. Wittstock, M. Bäumer, W. Dononelli, T. Klüner, L. Lührs, C. Mahr, L. V. Moskaleva, M. Oezaslan, T. Risse, A. Rosenauer, A. Staubitz, J. Weissmüller, A. Wittstock
Nanoporous Gold: From Structure Evolution to Functional Properties in Catalysis and Electrochemistry
Chem. Rev. 2023, 123, 6716-6792. Abstract & Link (open access)
Original Papers
- H. Kwon, H.-N. Barad, A. R. Silva Olaya, M. Alarcón-Correa, K. Hahn, G. Richter, G. Wittstock, P. Fischer
Ultra-pure Nanoporous Gold Films for Electrocatalysis
ACS Catal. 2023, 13, 11656-11665. Abstract & Link (open access) - H. Kwon, H.-N. Barad, A. R. Silva Olaya, M. Alarcón-Correa, K. Hahn, G. Richter, G. Wittstock, Peer Fischer
Dry Synthesis of Pure and Ultrathin Nanoporous Metallic Films
Appl. Mater. Interfaces 2023, 15, 5620-5627. Abstract & Link (open access) - A. R. Silva Olaya, F. Kühling, C. Mahr, B. Zandersons, A. Rosenauer, J. Weissmüller, G. Wittstock
Promoting Effect of the Residual Silver on the Electrocatalytic Oxidation of Methanol and Its Intermediates on Nanoporous Gold
ACS Catal. 2022, 12, 4415-4429. Abstract & Link - A. R. Silva Olaya, B. Zandersons, G. Wittstock
Effect of the Residual Silver and Adsorbed Lead Anions towards the Electrocatalytic Methanol Oxidation on Nanoporous Gold in Alkaline Media
Electrochim. Acta 2021, 383C, 138348. Abstract & LInk (Open Access) - A. R. Silva Olaya, B. Zandersons, G. Wittstock
Restructuring of nanoporous gold surface during electrochemical cycling in acidic and alkaline media. ChemElectroChem 2020, 7, 3670-3678. Abstract & Link (Open Access) - M. Haensch, M. Graf, W. Wang, A. Nefedov, C. Wöll, J. Weissmüller, G. Wittstock
Thermally-Driven Ag-Au Compositional Changes at the Ligament Surface in Nanoporous Gold: Implications for Electrocatalytic Applications
ACS Appl. Nano Mater. 2020, 3, 2197-2206. Abstract & Link - M. Haensch, L. Balboa, M. Graf, A. R. S. Olaya, J. Weissmüller, G. Wittstock
Mass Transport in Porous Electrodes Studied by Scanning Electrochemical Microscopy: Example of Nanoporous Gold
ChemElectroChem 2019, 6, 3160–3166. Abstract & Link (Open Access) - A. Lackmann, M. Bäumer, G. Wittstock, A. Wittstock
Independent control over structure size and residual silver content of nanoporous gold by galvanodynamically controlled dealloying
Nanoscale, 2018, 10, 17166-17173. Abstract & Link - M. Graf, M. Haensch, J. Carstens, G. Wittstock, J. Weissmüller
Electrocatalytic Methanol Oxidation with Nanoporous Gold: Microstructure and Selectivity
Nanoscale 2017, 9, 17839-17848. Abstract & Link - M. Hänsch, J. Behnken, L. Balboa, A. Dyck, G. Wittstock
Redox Titration of Gold and Platinum Surface Oxides at Porous Microelectrodes
Phys. Chem. Chem. Phys. 2017, 19, 22915-22925, Abstract & Link

