Interphases and Interfaces in Batteries
Contact
Mailing Address
Visitors
Interphases and Interfaces in Batteries
Scanning Electrochemical Microscopy at Battery Electrodes
Marius Muhle
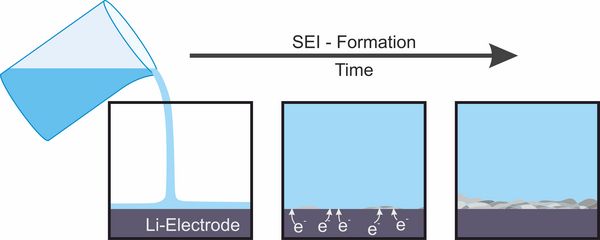
In lithium-ion or lithium-metal batteries the electrode potential is so low that electrons can be transferred to the molecules of the electrolyte which is located between both electrodes. The electrolyte usually is an organic liquid with hydrophobic ions. Its components can be reduced by electron transfer from the negative electrode forming solid reaction products that arrange as a thin interfacial layer (interphase) between the electrolyte and the negative electrode which is named solid electrolyte interphase (SEI). Due to its low electron conductivity, this layer stops an ongoing reduction of electrolyte components. In contrast, its high ionic conductivity still allows for the transfer of lithium ions that is needed for the battery cell to work. The solid electrolyte interphase is dynamic and can be destroyed by several processes. Mostly it is rebuilt afterwards. During such a process electrolyte is consumed which eventually can result in cell failure. The exact properties of the solid electrolyte interphase depends in a complex way on the electrode material, on its pretreatment, on the electrolyte components and on the operating conditions of the battery. The development of battery components to ensure a stable and durable solid electrolyte interphase for varying operating conditions is an opportunity to improve cycle life and safety of rechargeable batteries.[2,3]
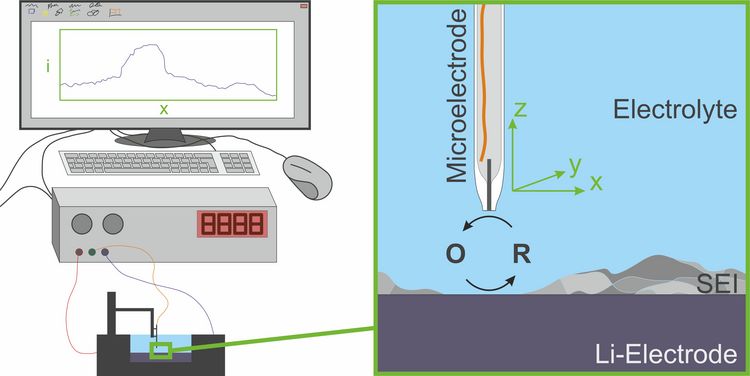
Scanning electrochemical microscopy (SECM) is one possible method for investigation of the properties of the solid electrolyte interphase. For this, a microelectrode can be moved in the electrolyte in all three spatial directions above the electrode that is covered by the interfacial layer. A mediator (i.e. an electron shuttle compound) is oxidized at the microelectrode and reduced at the sample, i.e. the negative electrode of the battery. As the solid electrolyte interphase inhibits electron transfer reactions between the electrode and the electrolyte components, the electric current at the microelectrode is hindered above the covered electrode. By measuring the current when the microelectrode is at different places, a two dimensional map can be drawn for the electron transfer blocking properties of the solid electrolyte interphase. Repeating these measurements after certain time steps enables a study of time-related development of the blocking behavior of the solid electrolyte interphase. By applying a current on the sample electrode, the response of the interfacial layer on charging and discharging processes can be investigated. The main advantage of scanning electrochemical microscopy in contrast to other common methods is the investigation of electrode surfaces inside their electrolyte environment without damaging them.
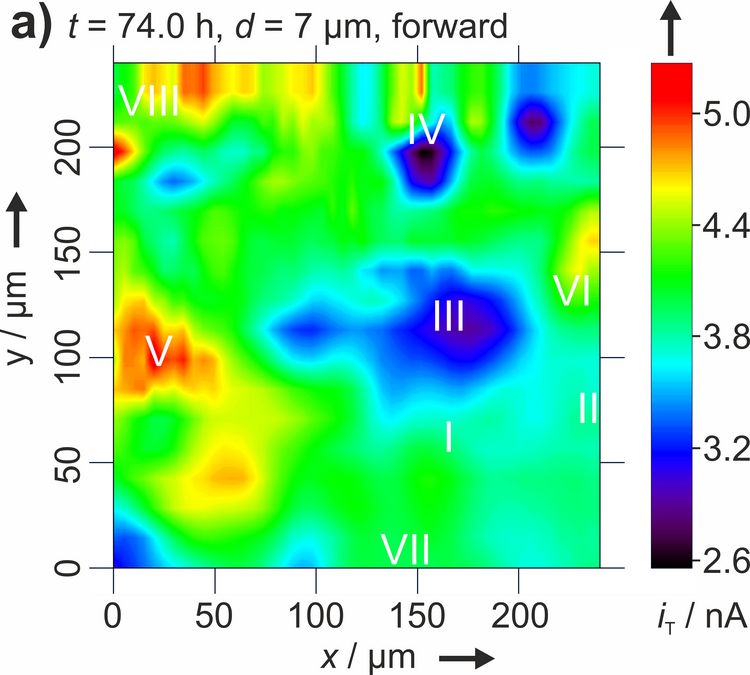
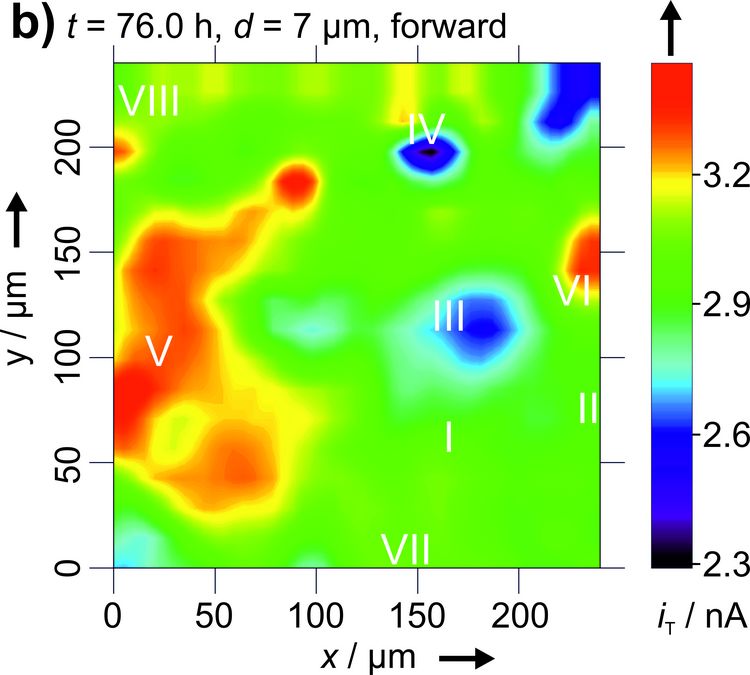
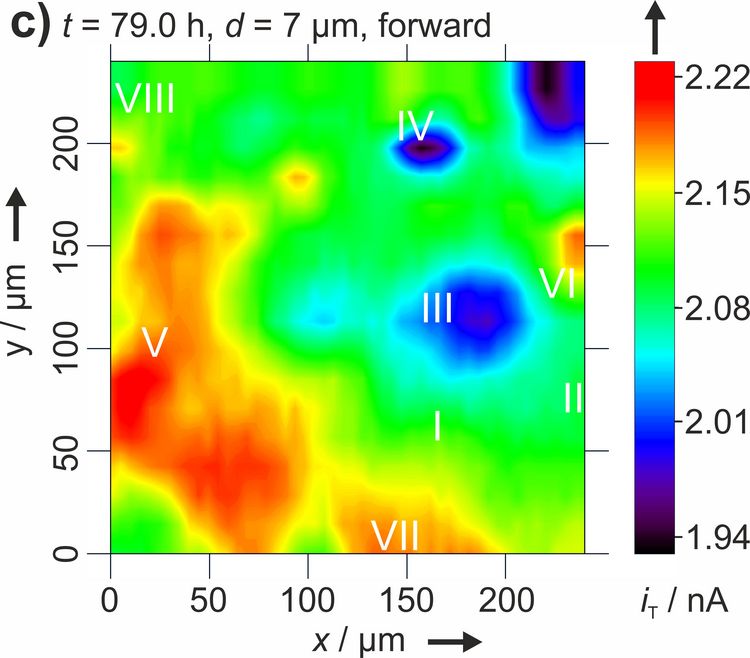
Figure 3: Two dimensional maps of the blocking behavior of a solid electrolyte interphase on graphite over time. Reproduced with permission from [4]. Copyright 2016 The Authors.
Further Reading
[1] T. B. Reddy, Linden’s handbook of batteries. McGraw-Hill Education 2011.
[2] X.-B. Cheng, R. Zhang, C.-Z. Zhao, F. Wei, J.-G. Zhang, Q. Zhang; A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. https://doi.org/10.1002/advs.201500213.
[3] S. K. Heiskanen, J. Kim, B. L. Lucht; Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. https://doi.org/10.1016/j.joule.2019.08.018.
Own Contribution to the Research Area
B. Krueger, K. K. Rücker, G. Wittstock
Redox Mediators for Faster Lithium Peroxide Oxidation in a Lithium-Oxygen Cell: A Scanning Electrochemical Microscopy Study
ACS Appl. Energy Mater. 2022, 5, 3724-3733. Abstract & Link
P. Bärmann, B. Krueger, S. Casino, M. Winter, T. Placke, G. Wittstock
Impact of the Crystalline Li15Si4 Phase on the Self Discharge Mechanism of Silicon Negative Electrodes in Organic Electrolytes on Single Crystalline Silicon Investigated by Scanning Electrochemical Microscopy. ACS Appl. Energy Mater. 2019, 2, 1388–1392. https://doi.org/10.1021/acsaem.8b01967.
ACS Appl Mater. Interfaces 2020, 12, 55903-55912. Abstract & Link
B. Krueger, L. Balboa, J. F. Dohmann, M. Winter, P. Bieker, G. Wittstock
Solid Electrolyte Interphase Evolution on Li Metal Electrodes Followed by Scanning Electrochemical Microscopy Under Realistic Battery Cycling Current Densities
ChemElectroChem 2020, 7, 3590-3596. Abstract & Link (Open Access)
P. Schwager, H. Bülter, I. Plettenberg, G. Wittstock
Review of Local In Situ Probing of Interfaces in Lithium-Ion and Lithium-Oxygen Batteries [review].
Energy Technol. (Weinheim, Ger.) 2016, 4, 1472-1485, Abstract & Link (Open Access)
[4] H. Bülter, F. Peters, G. Wittstock
Scanning Electrochemical Microscopy for the In Situ Characterization of Solid Electrolyte Interphases: Highly Oriented Pyrolytic Graphite vs. Graphite Composite
Energy Technol. (Weinheim, Ger.) 2016, 4, 1486-1494. Abstract & Link (Open Access)
H. Bülter, P. Schwager, D. Fenske, G. Wittstock
Observation of Dynamic Interfacial Layers in Li-Ion and Li-O2 Batteries by Scanning Electrochemical Microscopy
Electrochim. Acta 2016, 199, 366-379. Abstract & Link
H. Bülter, M. Sternad, E. dos Santos Sardinha, J. Witt, C. Dosche, M. Wilkening, G. Wittstock
Investigation of the Electron Transfer at Si Electrodes: Impact and Removal of the Native SiO2 Layer
J. Electrochem. Soc. 2016, 163(3), A504-A512. Abstract & Link
H. Bülter, F. Peters, J. Schwenzel, G. Wittstock
In Situ Quantification of the Swelling of Graphite Composite Electrodes by Scanning Electrochemical Microscopy
J. Electrochem. Soc. 2016, 163(2), A27-A34. Abstract & Link
H. Bülter, F. Peters, J. Schwenzel, G. Wittstock
Comparison of Electron Transfer Properties of the SEI on Graphite Composite and Metallic Lithium Electrodes by SECM at OCP
J. Electrochem. Soc. 2015, 13, A7024-A7036, Abstract & Link
H. Bülter, F. Peters, J. Schwenzel, G. Wittstock
Spatiotemporal Changes of the Solid Electrolyte Interphase in Lithium-Ion Batteries Detected by Scanning Electrochemical Microscopy
Angew. Chem. int. Ed. 2014, 126, 10699 –10704, Abstract & Link